Publications
Below is the list of publications by the Maes team. In many cases you have an option to access the full text of the publication at the journal website and to download it as a PDF file by clicking on the Full Text link. Please note that in order to be able to download a paper, you or your institution must have a subscription for the corresponding journal. Alternatively, please feel free to contact us for a reprint of the paper.
Currently, this list contains publications from 2016 onwards. A full list of publications can be found here.
2025
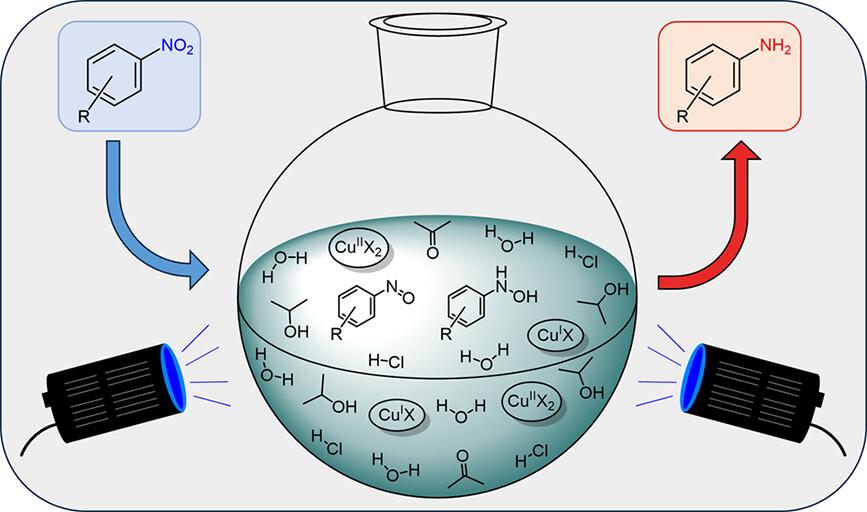
Visible Light-Photoinduced and Cu-Catalyzed Reduction of Nitrobenzenes into Anilines
M. Bal, W. Van Hoey, R. Cleirbaut, F. Lemière, S. Van Doorslaer, P. Cool, B. U. W. Maes, ACS Catal. 2025, 15, 4726–4738.
Unveiling the Potential of a Cobalt-Based Metal-Organic Framework in Carbodiimide Synthesis
D. S. Verdoorn, A. Zuliani, P. Ranjan, J. P. Holgado, N. Khiar, J. M. Saya, C. Carrillo-Carrión, B. U. W. Maes, R. V. A. Orru, Adv. Synth. Catal. 2025, e202401540. (ASAP)

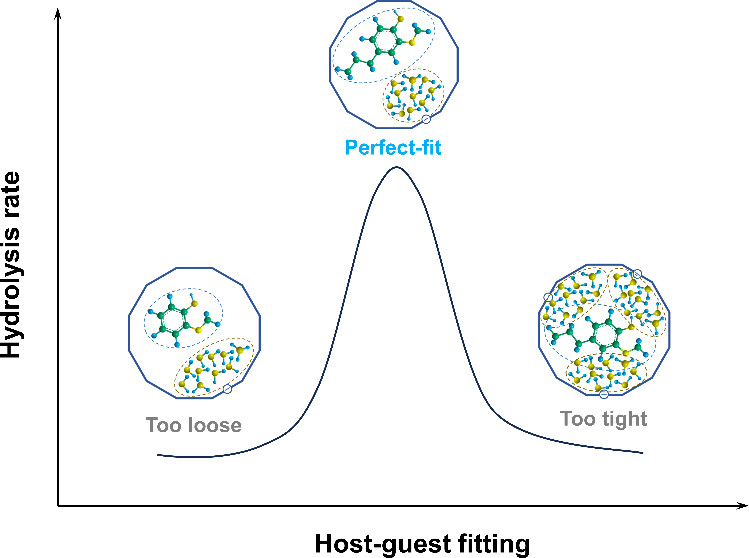
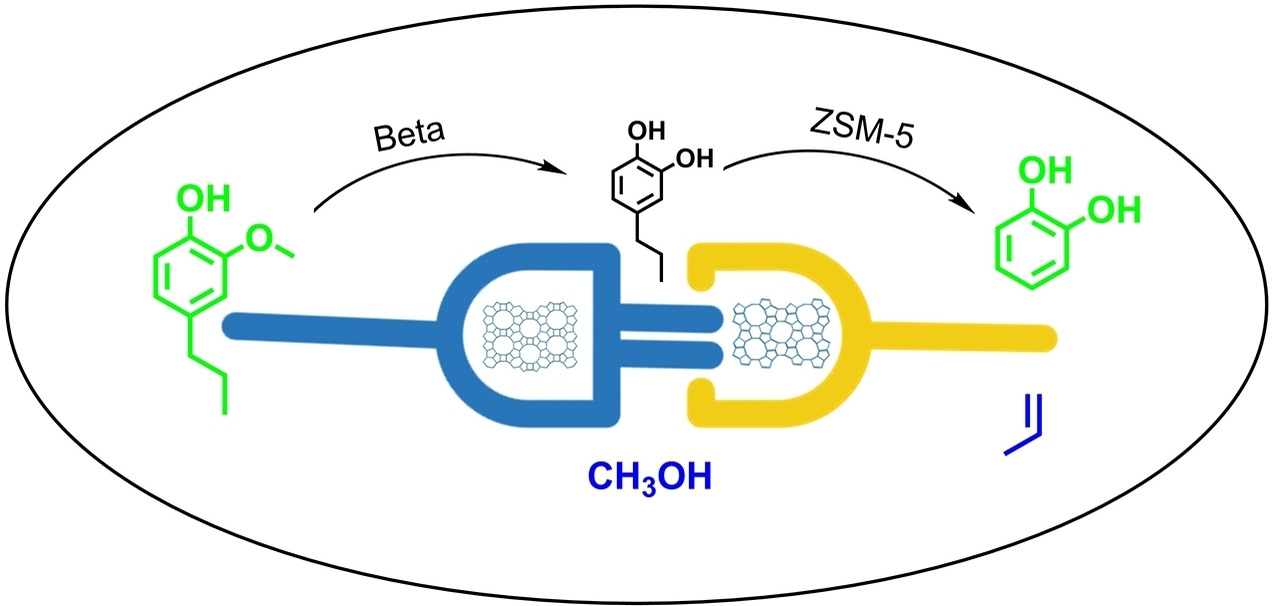
Spatial Scale Matters: Hydrolysis of Aryl Methyl Ethers over Zeolites
X. Wu, M. Bal, Q. Zhang, S.-T. Bai, I. Scodeller, W. Vermandel, J. Yu, B. U. W. Maes, B. F. Sels, J. Am. Chem. Soc. 2025,
6, 4915–4929.
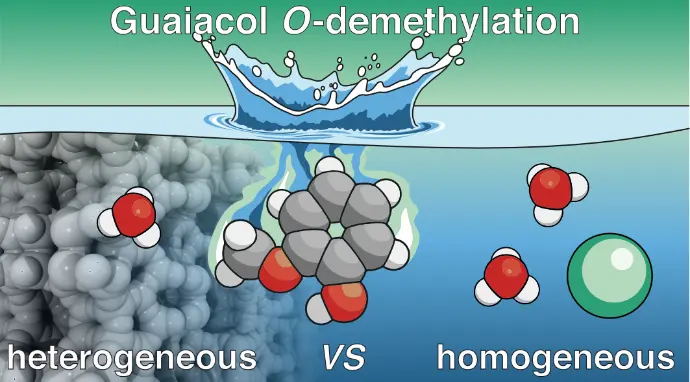
Confined hot-pressurized water in Brønsted-acidic beta zeolite speeds up the O-demethylation of guaiacol
M. Bocus, E. Van Den Broeck, X. Wu, M. Bal, J. Bomon, L. Vanduyfhuys, B. F. Sels, B. U. W. Maes, V. Van Speybroeck, Nat. Catal. 2025,
8, 33–45
.
Muconic Acid as Key Biorenewable Platform Molecule
C. Ver Elst, B. U. W. Maes, Encyclopedia of Green Chemistry, 2025.

2024
One-Pot Amide Synthesis via a Shelf-Stable and Renewable C1 Transfer Reactant
E. Renders, Z. Dobi, K. Weemaes, J. Bomon, C. Mensch, G. De Smet, N. R. Bheemireddy, W. Herrebout, B. U. W. Maes, ACS Sustainable Chem. Eng. 2024, 12, 15935–15947.
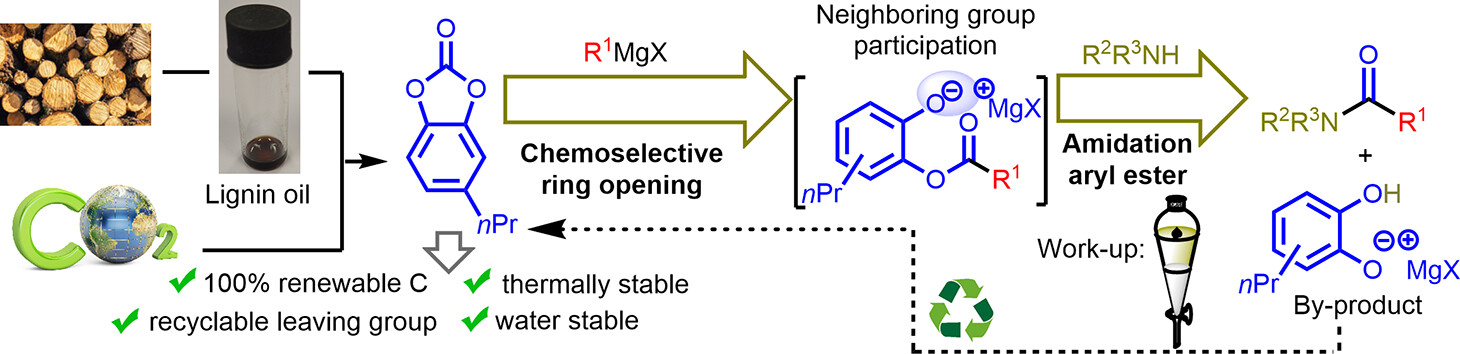
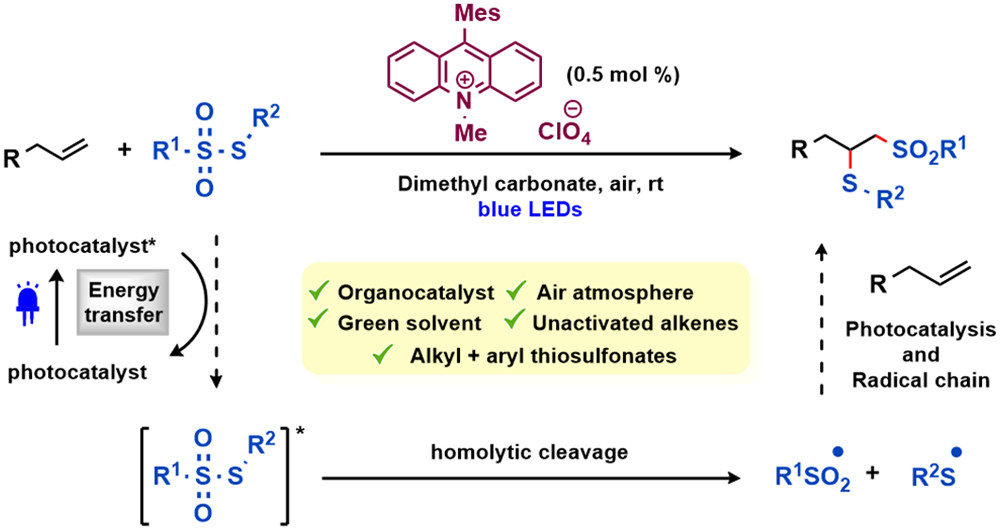
Hydrosulfonylation of Unactivated Alkenes Involving Sulfonyl Radical Generation via Photocatalytic Activation of Symmetrical Disulfones by an Energy Transfer Mimicry
D. De Vos, A. V. Cunha, B. Bongsuiru Jei, B. U. W. Maes, ACS Catal. 2024, 14, 12282–12296.
The paper was featured in Org. Chem. Highlights: Reactions of Alkene!
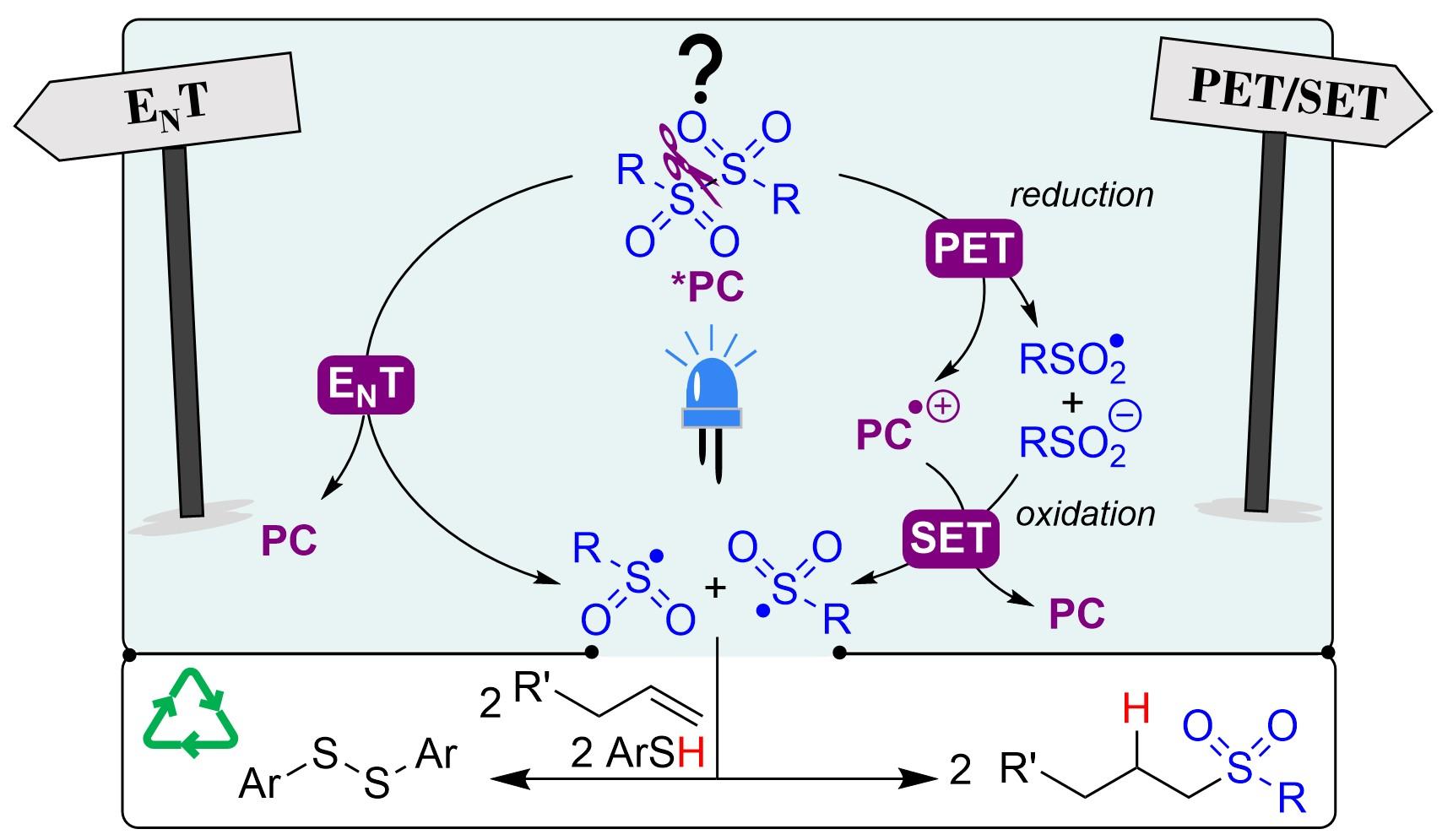
Rhodium-Catalyzed Direct ortho-Arylation of Anilines
C. Jacob, J. Annibaletto, J. Peng, R. Bai, B. U. W. Maes, Y. Lan, G. Evano, Angew. Chem. Int. Ed. 2024, 63, e202403553.
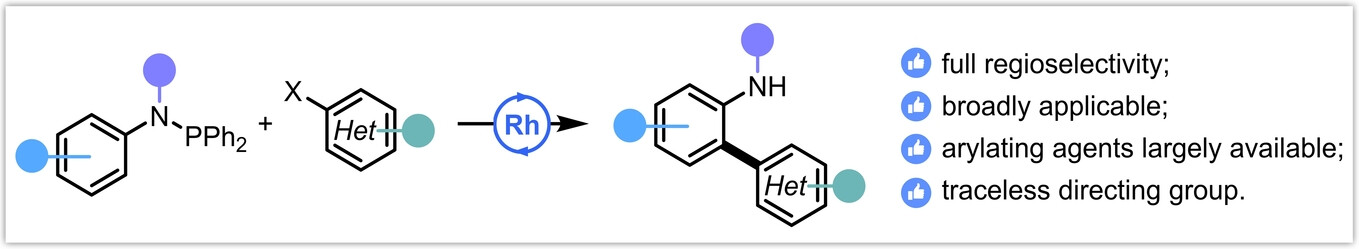
Selective C(aryl)–O bond cleavage in biorenewable phenolics
G. De Smet, X. Bai, B. U. W. Maes, Chem. Soc. Rev. 2024, 53, 5489–5551.
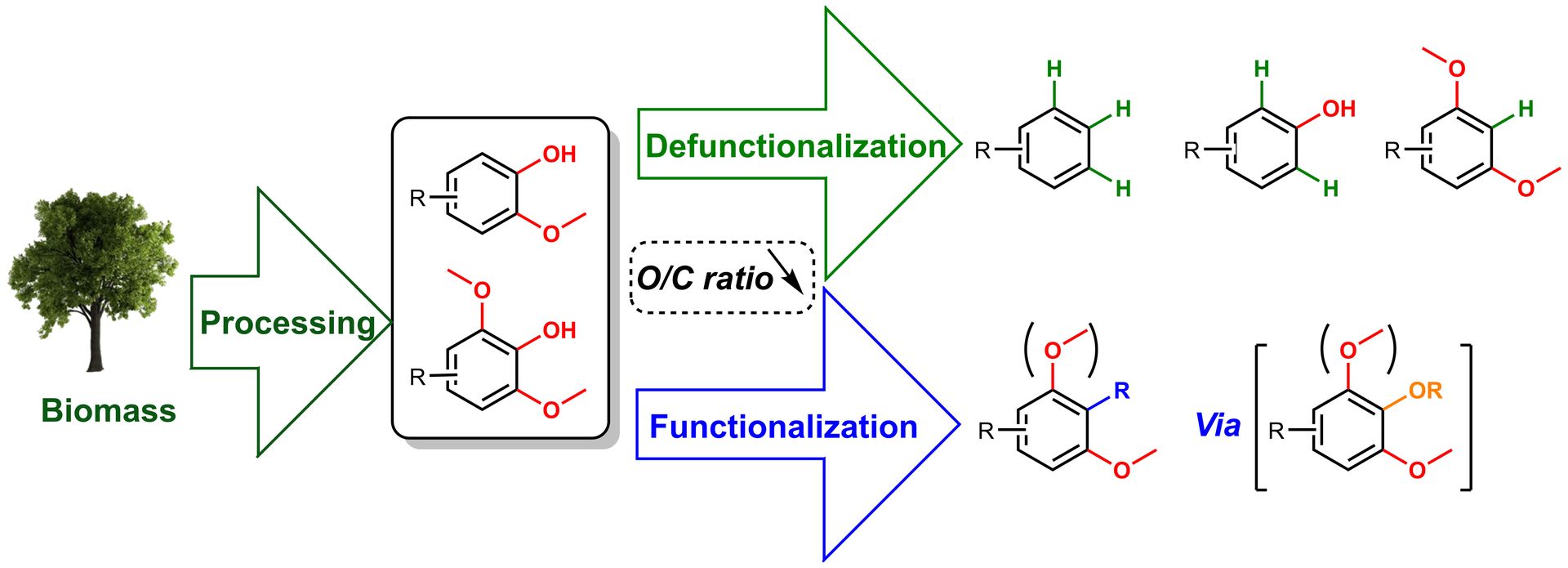
Advancements and Perspectives toward Lignin Valorization via O-Demethylation
X. Wu, E. Smet, F. Brandi, D. Raikwar, Z. Zhang, B. U. W. Maes, B. F. Sels, Angew. Chem. Int. Ed. 2024, e202317257.
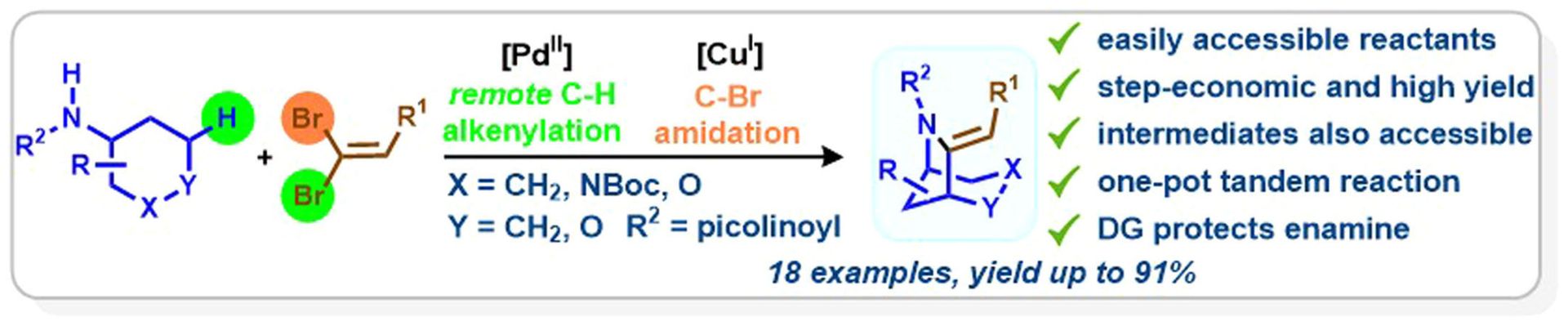
Directed Palladium-Catalyzed γ-C(sp³)–H Alkenylation of (Aza and Oxa) Cyclohexanamines with Bromoalkenes: Bromide Precipitation as an Alternative to Silver Scavenging
K. Gadde, N. R. Bheemireddy, J. Heitkämper, A. Nova, B. U. W. Maes, ACS Catal. 2024, 14, 1157–1172.
2023
(Het)Arene/Alkene Cross-Dehydrogenative Coupling for C(sp²)-C(sp²) Bond Formation
C. Sambiagio, B. U. W. Maes, Science of Synthesis: Cross-Dehydrogenative Coupling, Chapter 6, 2023, 119–152.

Dearomative Spirocyclization of Tryptamine-Derived Isocyanides via Iron-Catalyzed Carbene Transfer
T. Roose, F. McSorley, B. Groenhuijzen, J. M. Saya, B. U. W. Maes, R. V. A. Orrù, E. Ruijter, J. Org. Chem. 2023, 88,
17345–17355.
Synthesis of Levulinic Acids From Muconic Acids in Hot Water
C. Ver Elst, R. Vroemans, M. Bal, S. Sergeyev, C. Mensch, B. U. W. Maes, Angew. Chem. Int. Ed. 2023, e202309597.
A Cobalt Mediated Nitrene Transfer aza-Wittig Cascade Reaction To Access 1,3,4-Oxadiazole Scaffolds
D.S. Verdoorn, P. Ranjan, T. de Reuver, E. Janssen, C. M. L. Vande Velde, J.M. Saya, B. U. W. Maes, R. V. A. Orru, Org. Lett., 2023, 25, 4005–4009.
Direct Arylation of C(sp²)–H Bonds in Anilines
C. Jacob, J. Annibaletto, B. U. W. Maes, G. Evano, Synthesis 2023, 55, 1799–1823.
Iron-Catalysed Carbene Transfer to Isocyanides as a Platform for Heterocycle Synthesis
T. R. Roose, H. D. Preschel, H. M. Tejedor, J. C. Roozee, T. A. Hamlin, B. U. W. Maes, E. Ruijter, R. V. A. Orru, Chem. Eur. J. 2023, 29, e202203074.
Emerging Activation Modes and Techniques in Visible-Light Photocatalyzed Organic Synthesis
D. De Vos, K. Gadde, B. U. W. Maes, Synthesis 2023, 55, 193–231.
Basic Concepts and Activation Modes in Visible-Light Photocatalyzed Organic Synthesis
K. Gadde, D. De Vos, B. U. W. Maes, Synthesis 2023, 55, 164–192.
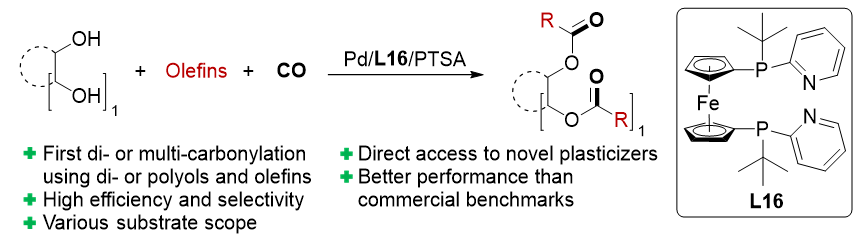
Efficient Synthesis of Novel Plasticizers by Direct Palladium-catalyzed Di- or Multi-carbonylations
Y. Hu, R. Sang, R. Vroemans, G. Mollaert, R. Razzaq, H. Neumann, H. Junge, R. Franke, R. Jackstell, B. U. W. Maes, M. Beller, Angew. Chem. Int. Ed. 2023, e202214706.
2022
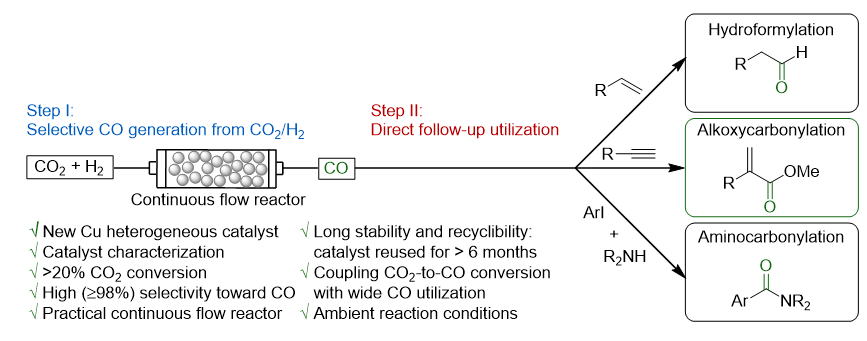
A practical concept for catalytic carbonylations using carbon dioxide
R. Sang, Y. Hu, R. Razzaq, G. Mollaert, H. Atia, U. Bentrup, M. Sharif,
H. Neumann, H. Junge, R. Jackstell, B. U. W. Maes, M. Beller, Nat. Commun. 2022, 13, 4432.
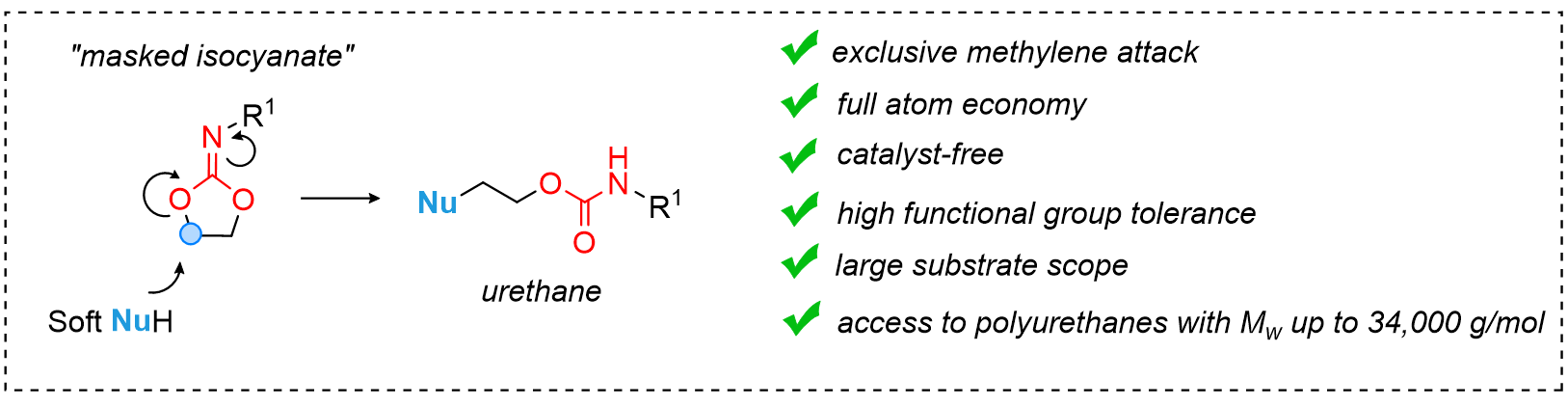
Functional Regioregular (Poly)urethanes from Soft Nucleophiles and Cyclic Iminocarbonates
B. Grignard, P. Mampuys, J. Escudero, D. Masullo, F. Lemière, B. U. W. Maes,
C. Detrembleur, Polymer Chemistry, 2022, 13, 6599–6605.
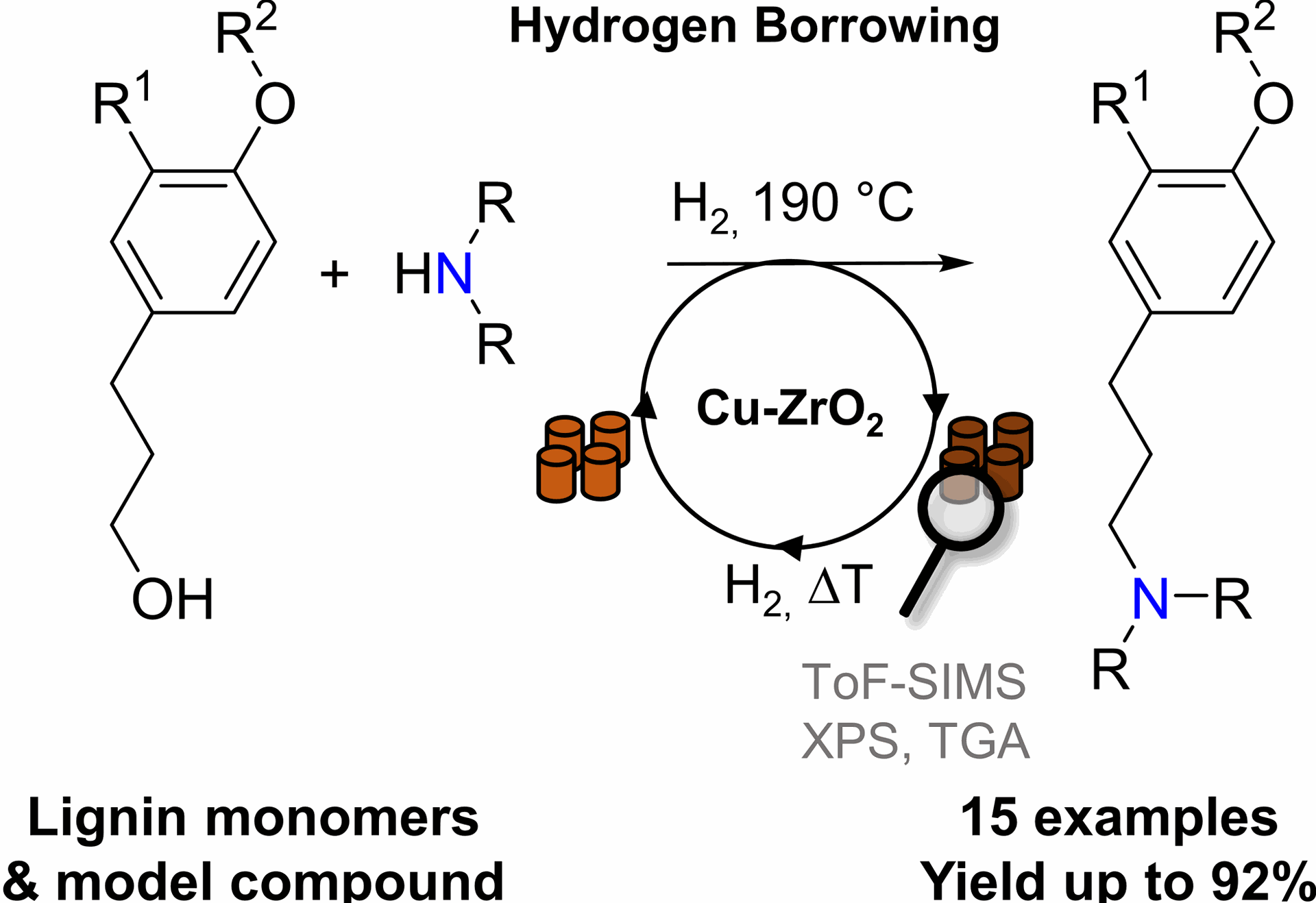
Hydrogen borrowing: Towards aliphatic tertiary amines from lignin model compounds using a supported copper catalyst
D. Ruijten, T. Narmon, H. De Weer, R. van de Zweep, C. Poleunis, D. P. Debecker, B. U. W. Maes, B. F. Sels, ChemSusChem 2022, 15, e202200868.
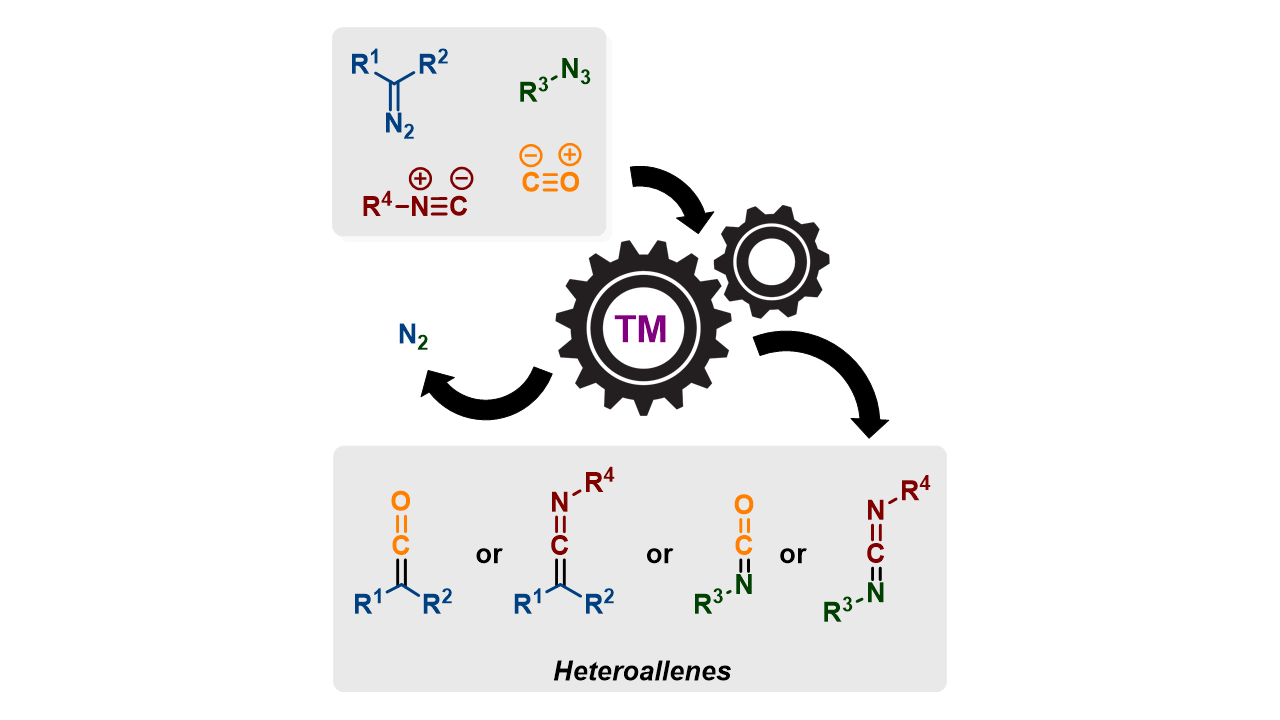
Transition metal-catalysed carbene- and nitrene transfer to carbon monoxide and isocyanides
T. R. Roose, D. S. Verdoorn, P. Mampuys, E. Ruijter, B. U. W. Maes, R. V. A. Orru, Chem. Soc. Rev. 2022, 51, 5842–5877.
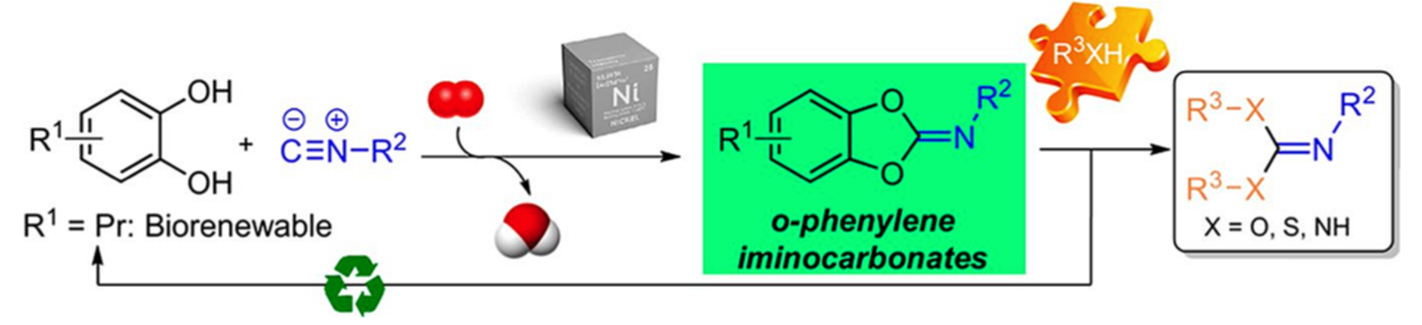
Synthesis of Heterocycles via Aerobic Ni-Catalyzed Imidoylation of Aromatic 1,2-Bis-nucleophiles with Isocyanides
J. Escudero, P. Mampuys, C. Mensch, C. B. Bheeter, R. Vroemans, R. V. A. Orru, J. Harvey, B. U. W. Maes, ACS Catal. 2022, 12, 6857–6873.
Selective Nickel-Catalyzed Hydrodeacetoxylation of Aryl Acetates
G. De Smet, X. Bai , C. Mensch, S. Sergeyev, G. Evano, B. U. W. Maes, Angew. Chem. Int. Ed. 2022, 61, e202201751.
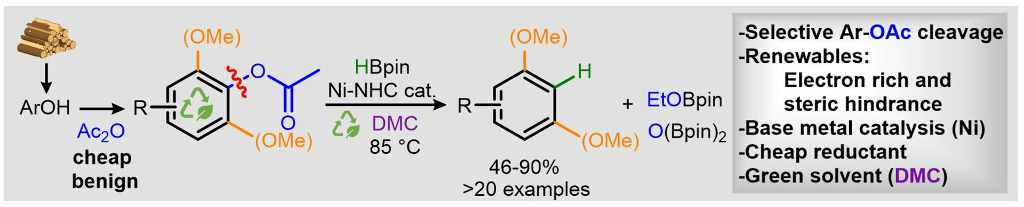
This manuscript was selected as Hot Paper!
Lignin-First Monomers to Catechol: Rational Cleavage of C-O and C-C Bonds over Zeolites
X. Wu, Y. Liao, J. Bomon, G. Tian, S.-T. Bai, K. Van Aelst, Q. Zhang, W. Vermandel, B. Wambacq,
B. U. W. Maes, J. Yu, B. F. Sels, ChemSusChem 2022, 15, e202102248.
2021
Expedient synthesis of bridged bicyclic nitrogen scaffolds via orthogonal tandem catalysis
S. Biswas, B. F. Van Steijvoort, M. Waeterschoot, N. R. Bheemireddy, G. Evano, B. U. W. Maes, Angew. Chem. Int. Ed. 2021, 60, 21988–21996.
This manuscript was selected as Hot Paper! The paper was furthermore highlighted in Synfacts 2021, 17, 1319 and featured in Org. Chem. Highlights: C-H functionalization!
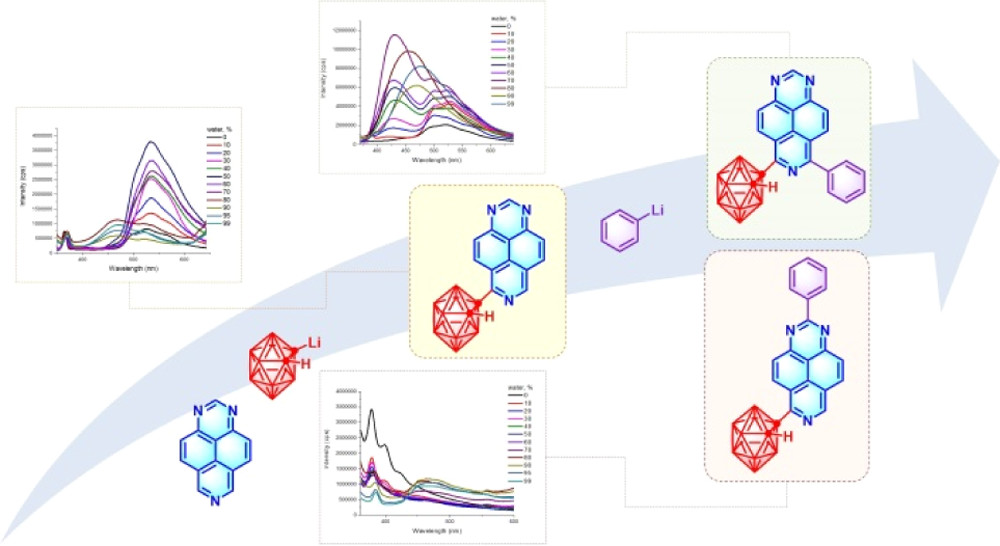
1,3,7-Triazapyrene-Based ortho-Carborane Fluorophores: Convenient Synthesis, Theoretical Studies, and Aggregation-Induced Emission Properties
L. A. Smyshliaeva, M. V. Varaksin, E. I. Fomina, M. V. Medvedeva, T. S. Svalova, A. N. Kozitsina, O. P. Demidov, I. V. Borovlev, C. Mensch, P. Mampuys, B. U. W. Maes, V. N. Charushin, O. N. Chupakhin, Organometallics 2021, 40, 2792–2807.
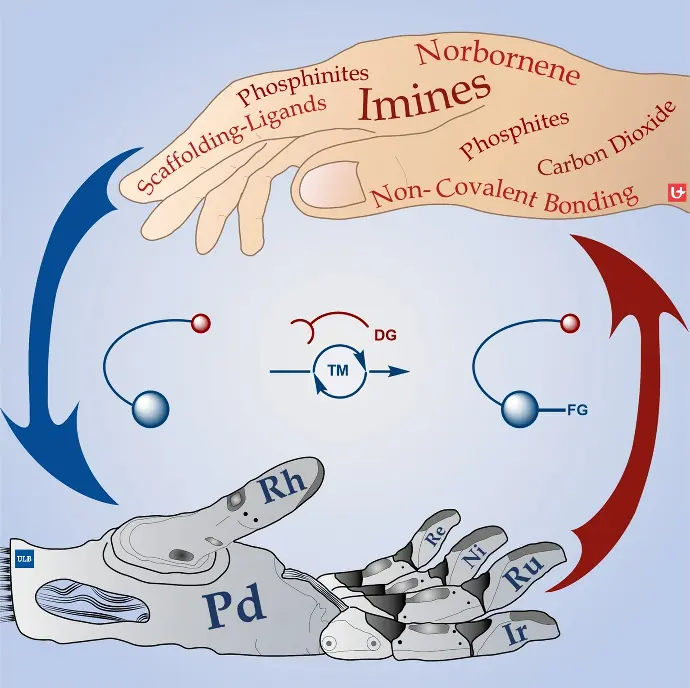
Transient Directing Groups in Metal-Organic Cooperative Catalysis
B. U. W. Maes, G. Evano, C. Jacob, Chem. Eur. J. 2021, 27, 13899–13952.
3-Substituted 2-isocyanopyridines as versatile convertible isocyanides for peptidomimetic design
C. Hollanders, M. Elsocht, O. Van der Poorten, M. Jida, E. Renders, B. U. W. Maes, S. Ballet. Chem. Commun. 2021, 57, 6863–6866.
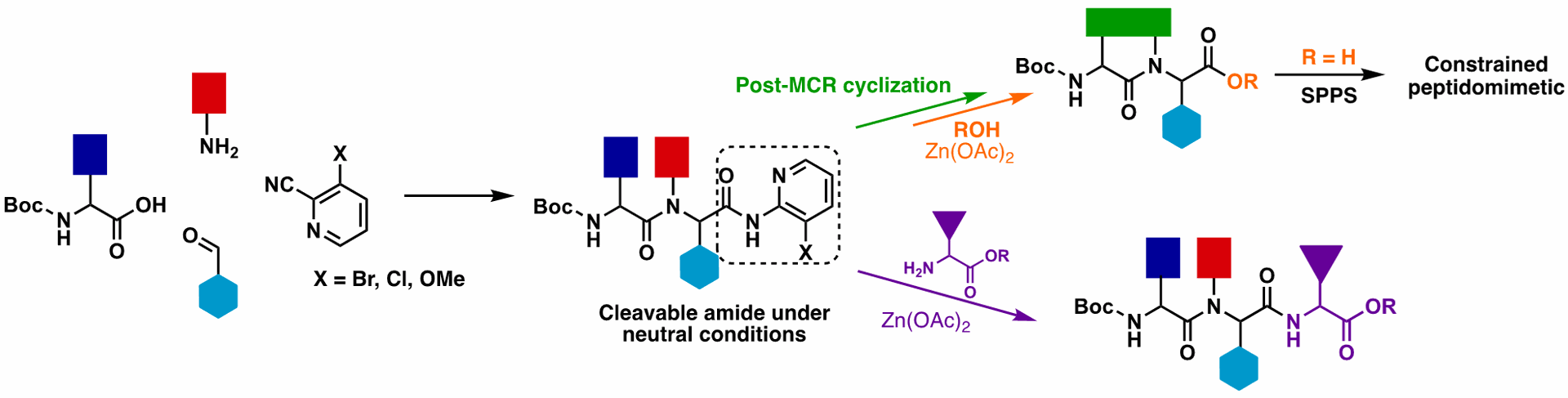
Homogeneous and heterogeneous catalysts for hydrogenation of CO₂ to methanol under mild conditions
S. Bai, G. De Smet, Y. Liao, R. Sun, C. Zhou, M. Beller, B. U. W. Maes, B. F. Sels, Chem. Soc. Rev. 2021, 50, 4259–4298.
Non-Directed Functionalization of Distal C(sp³)-H Bonds
C. Sambiagio, B. U. W. Maes, Remote C-H Bond Functionalizations: Methods and Strategies in Organic Synthesis, Chapter 12, 2021, 343–382.

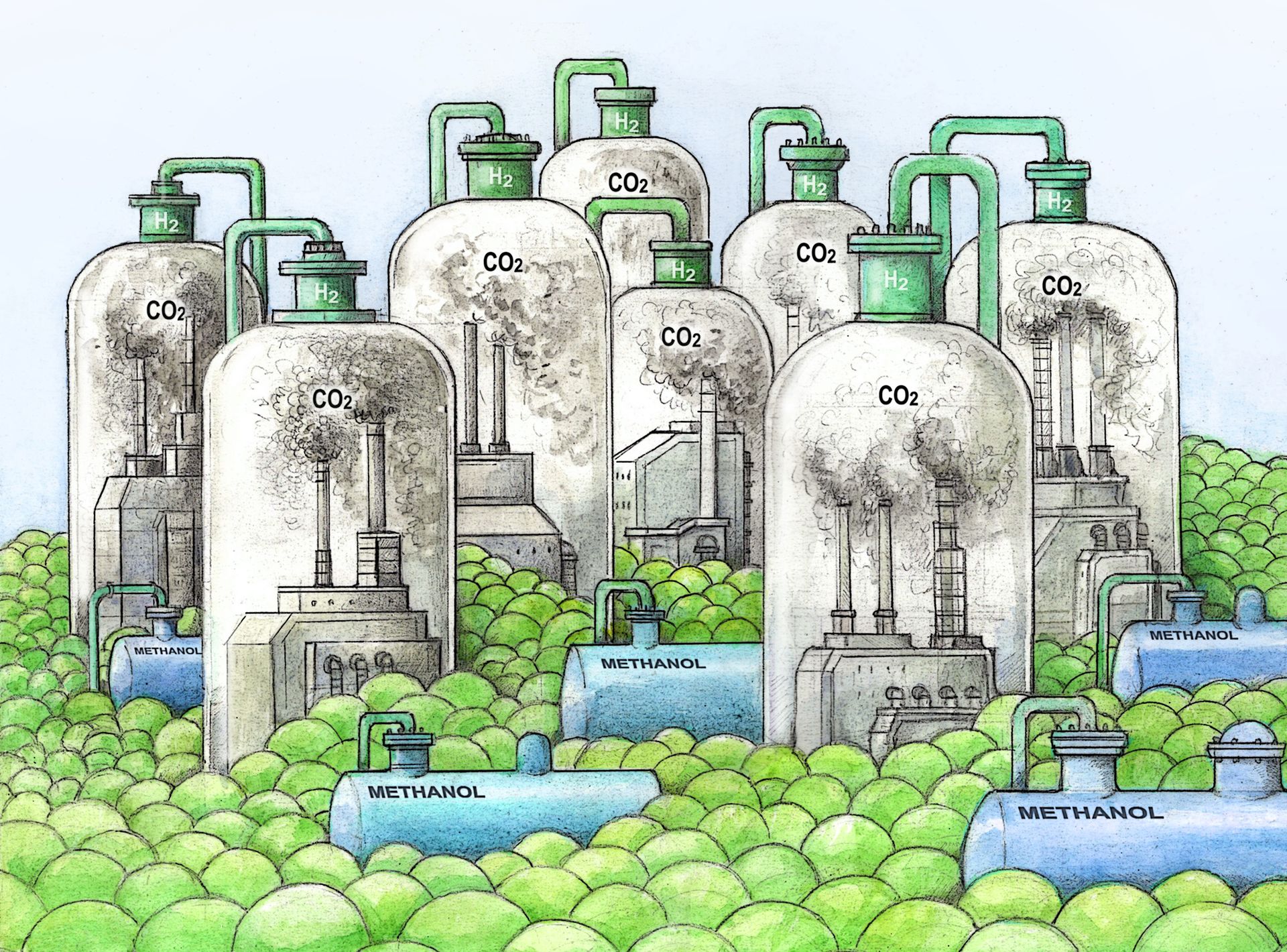
An Efficient Demethylation of Aromatic Methyl Ethers with HCl in Water
J. Bomon, M. Bal, T. K. Achar, S. Sergeyev, X. Wu, B. Wambacq, F. Lemière, B. Sels, B. U. Maes, Green Chem. 2021, 23, 1995–2009.
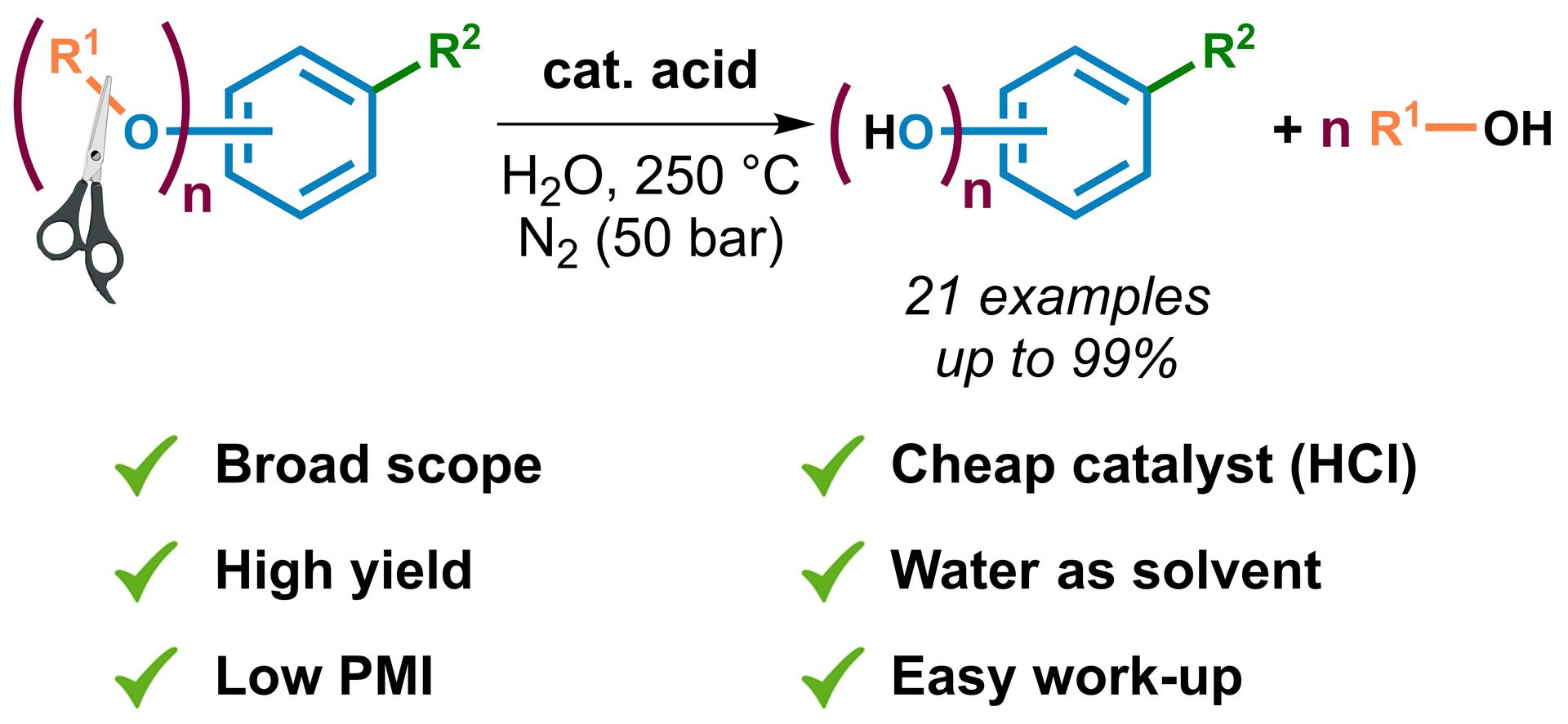
C(sp²)–H Functionalization in Non-Aromatic Azomethine-Based Heterocycles
A. A. Akulov, M. V. Varaksin, P. Mampuys, V. N. Charushin, O. N. Chupakhin, B. U. W. Maes, Org. Biomol. Chem. 2021, 19, 297–312.
Access to Biorenewable and CO₂-Based Polycarbonates from Exovinylene Cyclic Carbonates
F. Siragusa, E. Van Den Broeck, C. Ocando, A. J. Müller, G. De Smet, B. U. W. Maes, J. De Winter, V. Van Speybroeck, B. Grignard, C. Detrembleur, ACS Sustainable Chem. Eng. 2021, 9, 1714–1728.
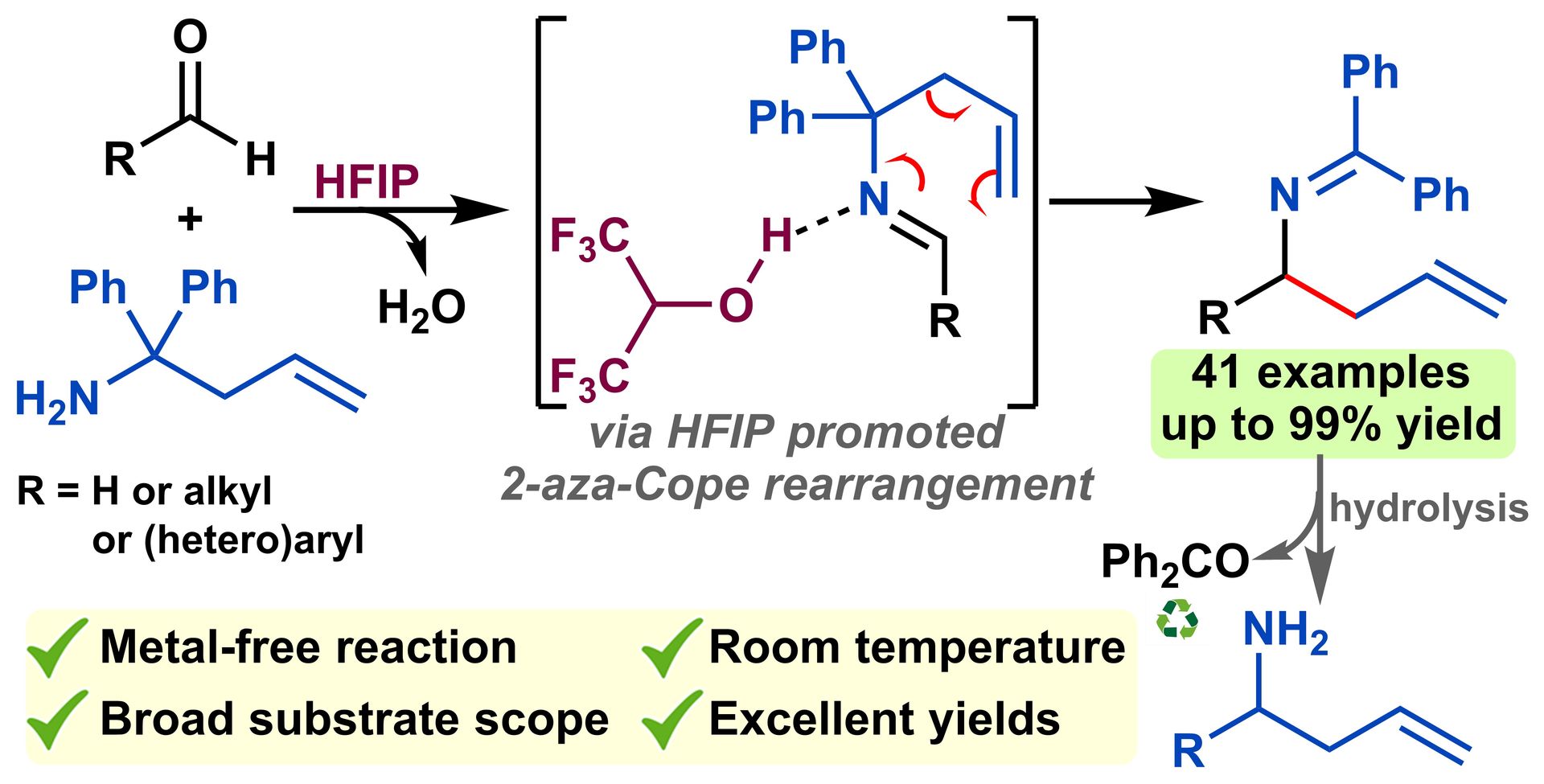
HFIP-mediated 2-aza-Cope rearrangement: metal-free synthesis of α-substituted homoallylamines at ambient temperature
K. Gadde, B. U. W. Maes, K. Abbaspour Tehrani, Org. Biomol. Chem. 2021, 19, 4067–4075.
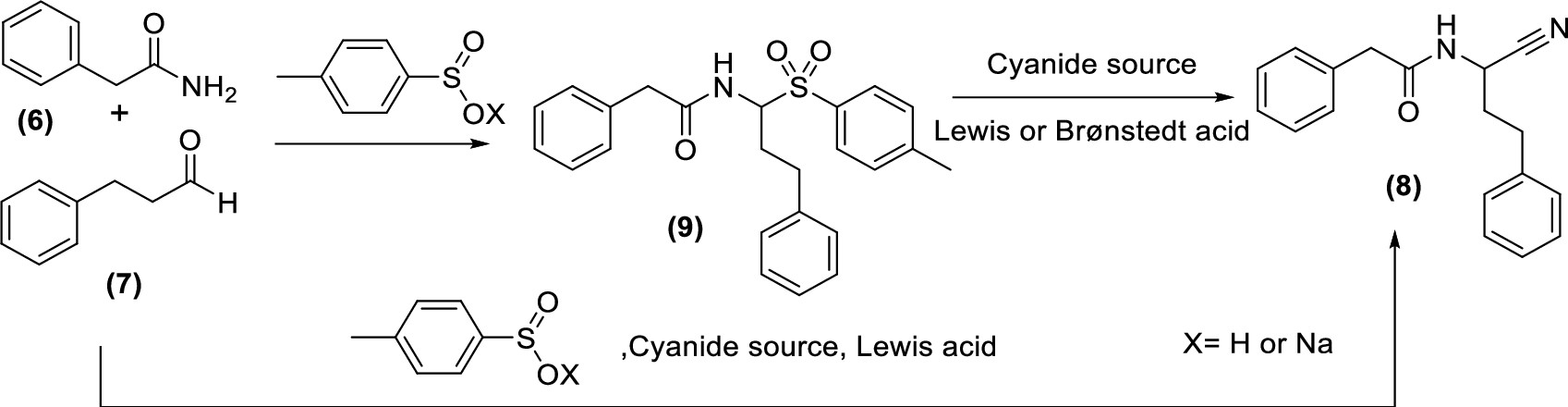
Strecker-Derived Methodology for Library Synthesis of N-Acylated α‑Aminonitriles
P. Goncalves, A. Peeraer, Y. Adriaenssens, L. Zonnekien, P. Franck, B. U. W. Maes, K. Augustyns, P. Van Der Veken, ACS Omega 2021, 6, 1328–1338
2020
Thiosulfonylation of Unactivated Alkenes with Visible-Light Organic Photocatalysis
K. Gadde, P. Mampuys, A. Guidetti, H. V. Ching, W. A. Herrebout, S. Van Doorslaer, K. Abbaspour Tehrani, B. U. W. Maes, ACS Catal. 2020, 10, 8765–8779.
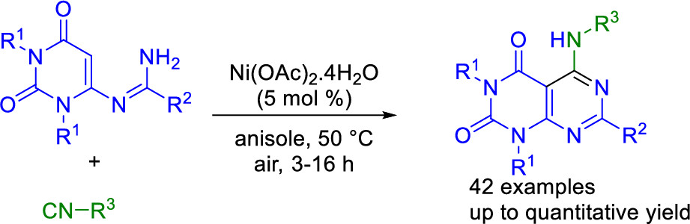
Synthesis of Densely Functionalized Pyrimidouracils by Nickel(II)-Catalyzed Isocyanide Insertion
J. W. Collet, B. Morel, H. Lin, T. R. Roose, P. Mampuys, R. V. A. Orru, E. Ruijter, B. U. W. Maes, Org. Lett. 2020, 22, 914–919.
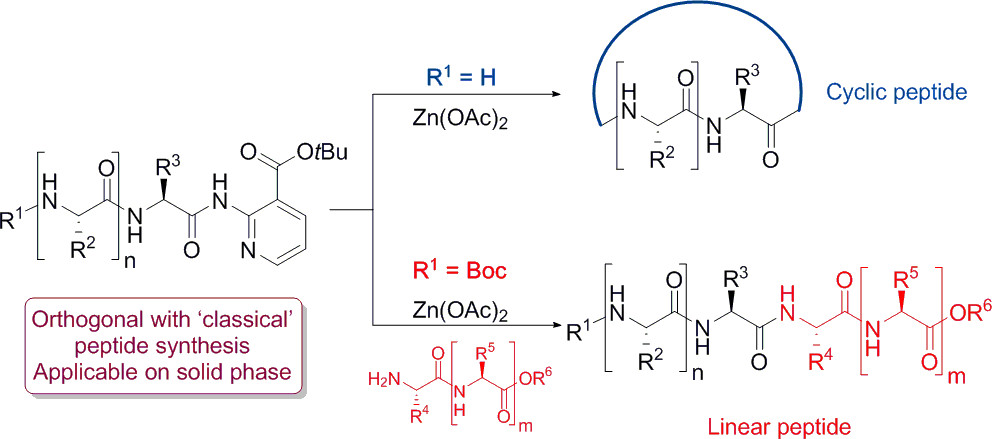
Zn-Catalyzed Nicotinate-Directed Transamidations in Peptide Synthesis
K. Hollanders, E. Renders, C. Gadais, D. Masullo, L. Van Raemdonck, C. C. D. Wybon, C. Martin, W. A. Herrebout, B. U. W. Maes, S. Ballet, ACS Catal. 2020, 10, 4280–4289.
This paper was highlighted in Synfacts 2020, 16, 0605.
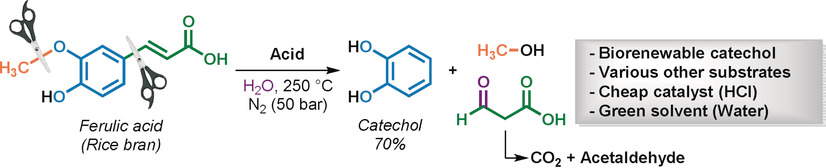
Brønsted Acid Catalyzed Tandem Defunctionalization of Biorenewable Ferulic acid and Derivates into Bio-catechol
J. Bomon, E. Van Den Broeck, M. Bal, Y. Liao, S. Sergeyev, V. Van Speybroeck, B. F. Sels, B. U. Maes, Angew. Chem. Int. Ed. 2020, 59, 3063–3068.
Base Metal-Catalyzed Isocyanide Insertions
J. W. Collet, T. R. Roose, E. Ruijter, B. U. W. Maes, R. V. A. Orru, Angew. Chem. Int. Ed. 2020, 59, 540–558.

Why we might be misusing Process Mass Intensity (PMI) and a methodology to apply it effectively as a discovery level metric
E. Monteith, P. Mampuys, L. Summerton, J. Clark, B. U. W. Maes, C. R. McElroy, Green Chem. 2020, 22, 123–135.

This work has been selected for Inclusion in the Measuring Green Chemistry: Methods, Models, and Metrics themed collection.
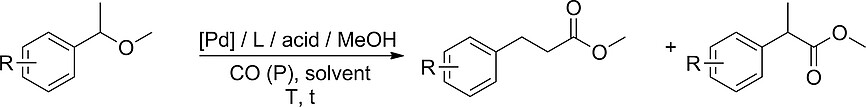
Palladium-Catalyzed Alkoxycarbonylation of sec-Benzylic Ethers
C. Schneider, R. Jackstell, B. U. W. Maes, M. Beller, Eur. J. Org. Chem. 2020, 2020, 932–936.
Synthesis – properties correlation and the unexpected role of the titania support on the Grignard surface modification
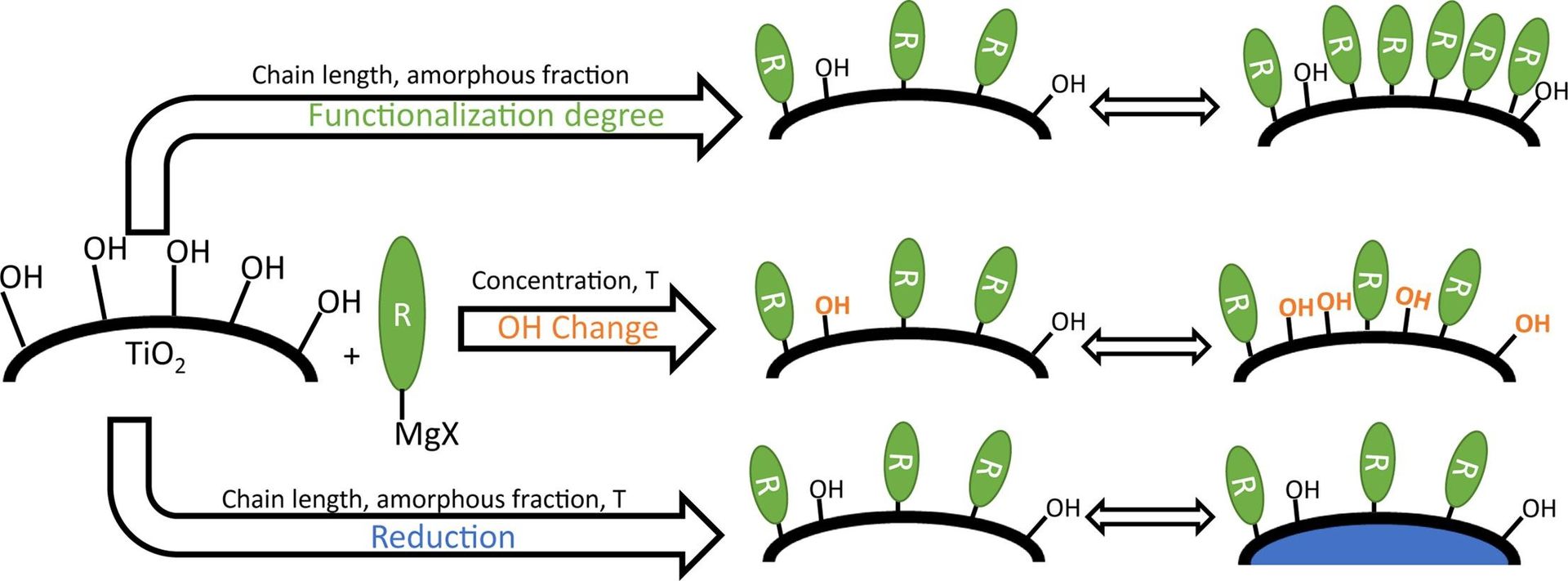
J. G. Van Dijck, P. Mampuys, H. V. Ching, D. Krishnan, K. Baert, T. Hauffman, J. Verbeeck, S. Van Doorslaer, B. U. W. Maes, M. Dorbec, A. Buekenhoudt, V. Meynen, Appl. Surf. Sci. 2020, 527, 146851.
The Non-innocent Role of Spin Traps in Monitoring Radical Formation in Copper-Catalyzed Reactions
M. Samanipour, H. Y. V. Ching, H. Sterckx, B. U. W. Maes, S. Van Doorslaer, Appl. Magn. Reson. 2020, 51, 1529–1542.
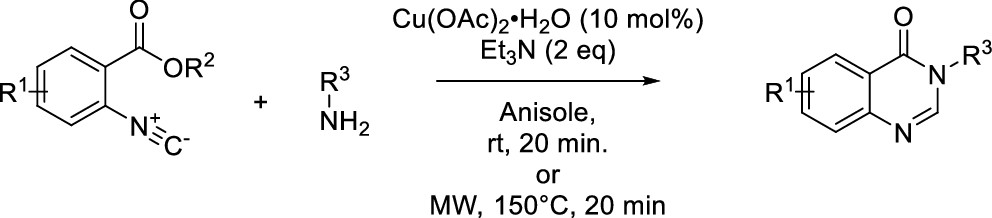
Synthesis of Quinazolin-4-ones by Copper-Catalyzed Isocyanide Insertion
J. W. Collet, E. A. van der Nol, T. R. Roose, B. U. W. Maes, R. V. A. Orru, J. Org. Chem. 2020, 85, 7378–7385.
2019
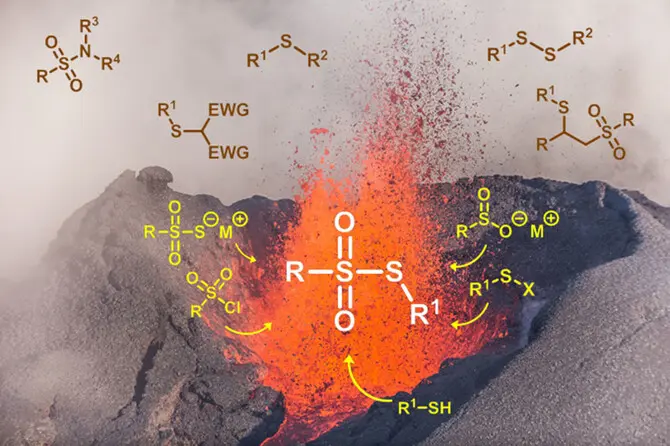
Thiosulfonates as Emerging Reactants: Synthesis and Applications
P. Mampuys, C. R. McElroy, J. H. Clark, R. V. A. Orru, B. U. W. Maes, Adv. Synth. Catal. 2020, 362, 3-64.
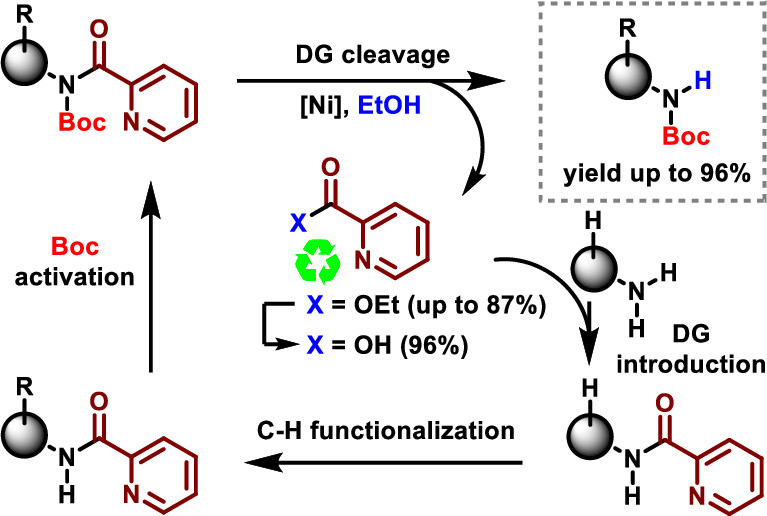
Directed C–H Functionalization Reactions with a Picolinamide Directing Group: Ni-Catalyzed Cleavage and Byproduct Recycling
S. Biswas, N. R. Bheemireddy, M. Bal, B. F. Van Steijvoort, B. U. W. Maes, J. Org. Chem. 2019, 84, 13112–13123.
Carbamate Synthesis Using a Shelf-Stable and Renewable C₁
Reactant
Z. Dobi, B. N. Reddy, E. Renders, L. Van Raemdonck, C. Mensch, G. De Smet, C. Chen, C. Bheeter, S. Sergeyev, W. A. Herrebout, B. U. W. Maes, ChemSusChem 2019, 12, 3103–3114.
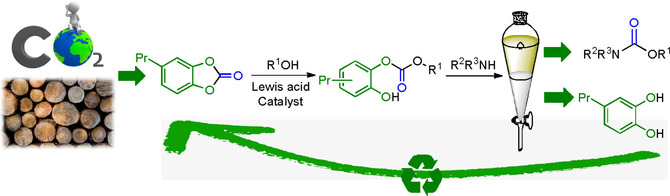
Lewis acidic FeCl₃
promoted 2-aza-Cope rearrangement to afford α-substituted homoallylamines in dimethyl carbonate
K. Gadde, J. Daelemans, B. U. W. Maes, K. Abbaspour Tehrani, RSC Adv. 2019, 9, 18013–18017.
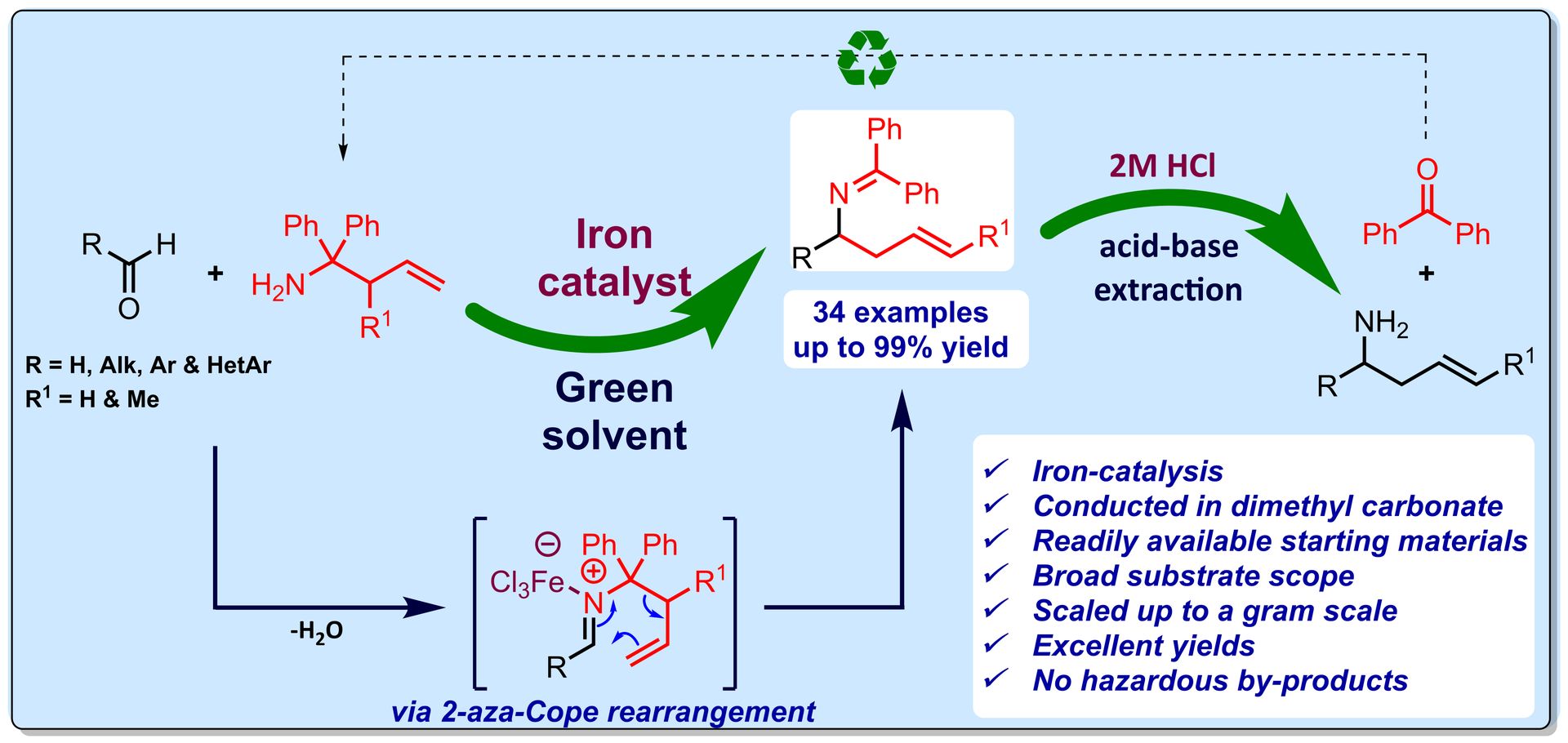
Catalytic Aerobic Oxidation of C(sp³)-H bonds
H. Sterckx, B. Morel, B. U. W. Maes, Angew. Chem. Int. Ed. 2019, 58, 7946–7970.
This review was highlighted in Org. Process Res. Dev. 2018, 22, 1036.
A New Wave of Amide Bond Formations for Peptide Synthesis
K. Hollanders, B. U. W. Maes, S. Ballet, Synthesis 2019, 51, 2261–2277.
Synthesis of Functionalized Pyrazin-2(1H)-ones via Tele-Nucleophilic Substitution of Hydrogen Involving Grignard Reactants and Electrophiles
P. Mampuys, T. D. Moseev, M. V. Varaksin, J. De Houwer, C. M. L. Vande Velde, O. N. Chupakhin, V. N. Charushin, B. U. W. Maes, Org. Lett. 2019, 21, 2699–2703.
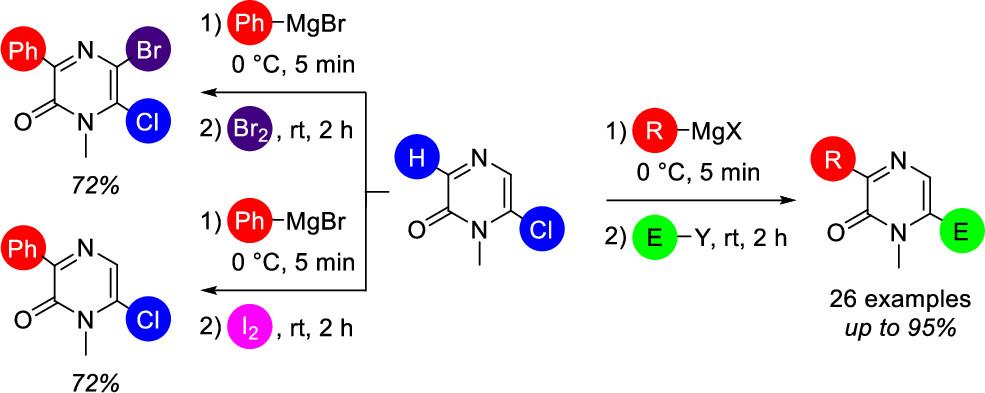
This paper was highlighted in Synfacts 2019, 15, 0757.
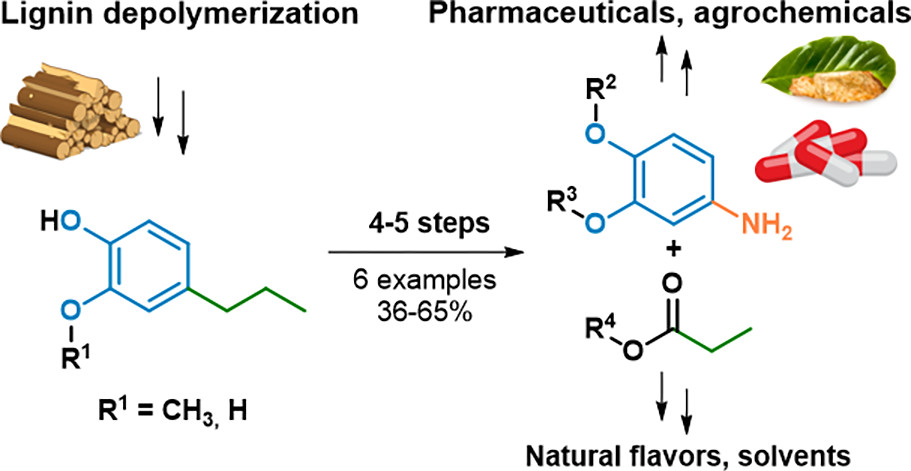
Bio-based aromatic amines from lignin-derived monomers
E. Blondiaux, J. Bomon, M. Smolen, N. Kaval, F. Lemiere, S. Sergeyev, L. Diels, B. F. Sels, B. U. W. Maes, ACS Sustainable Chem. Eng. 2019, 7, 6906–6916.
Ruthenium-Catalyzed Reductive Arylation of N-(2-Pyridinyl)amides with Isopropanol and Arylboronate Esters
T. O. Ronson, E. Renders, B. F. Van Steijvoort, X. Wang, C. C. D. Wybon, H. Prokopcová, L. Meerpoel, B. U. W. Maes, Angew. Chem. Int. Ed. 2019, 58, 482–487 .
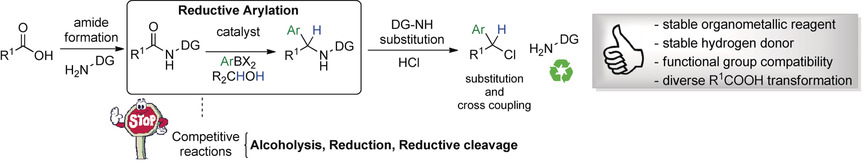
This paper was highlighted in Synfacts 2019, 15, 0168.
2018
Synthesis of Pd complexes containing tailed NHC ligands and their use in a Semi-continuous membrane assisted Suzuki cross coupling process.
D. Ormerod, M. Dorbec, E. Merkul, N. Kaval, N. Lefèvre, S. Hostyn, L. Eykens, J. Lievens, S. Sergeyev, B. U. W. Maes, Org. Process Res. Dev. 2018, 22, 1509–1517.
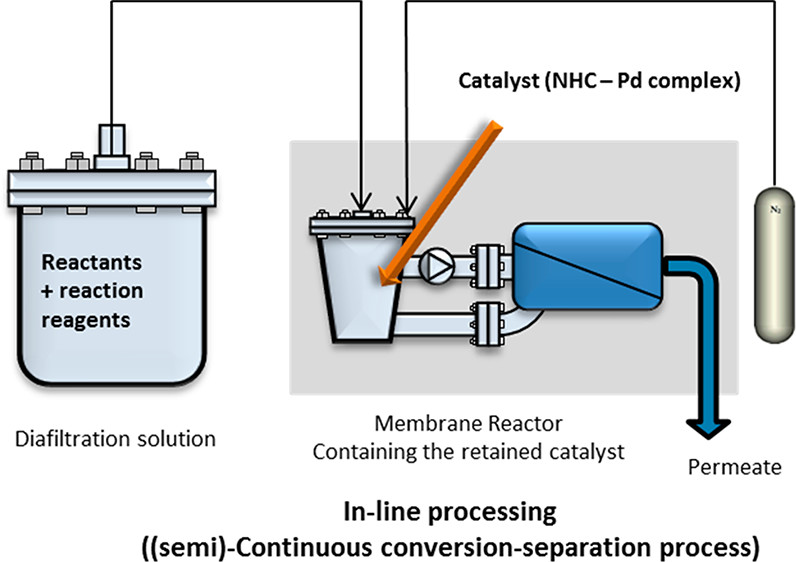
A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry
C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes, M. Schnürch, Chem. Soc. Rev. 2018, 47, 6603–6743.
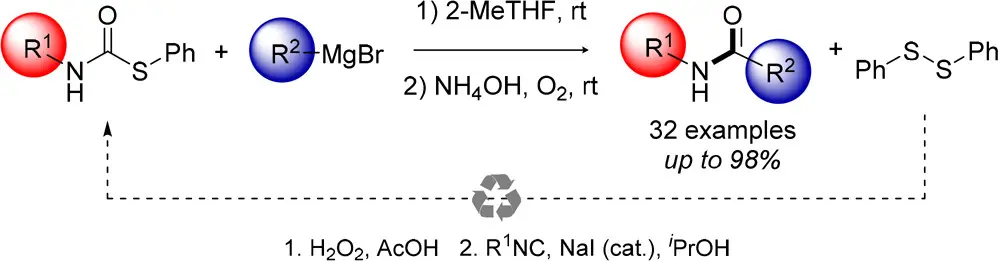
Synthesis of Secondary Amides from Thiocarbamates
P. Mampuys, E. Ruijter, R. V. A. Orru, B. U. W. Maes Org. Lett. 2018, 20, 4235–4239.
This publication was featured in Chemistry Views on 08/07/2018!
Zn-Catalyzed tert-Butyl Nicotinate-Directed Amide Cleavage as a Biomimic of Metallo-Exopeptidase Activity
C. C. D. Wybon, C. Mensch, K. Hollanders, C. Gadais, W. A. Herrebout, S. Ballet, B. U. W. Maes ACS Catal. 2018, 8, 203–218.
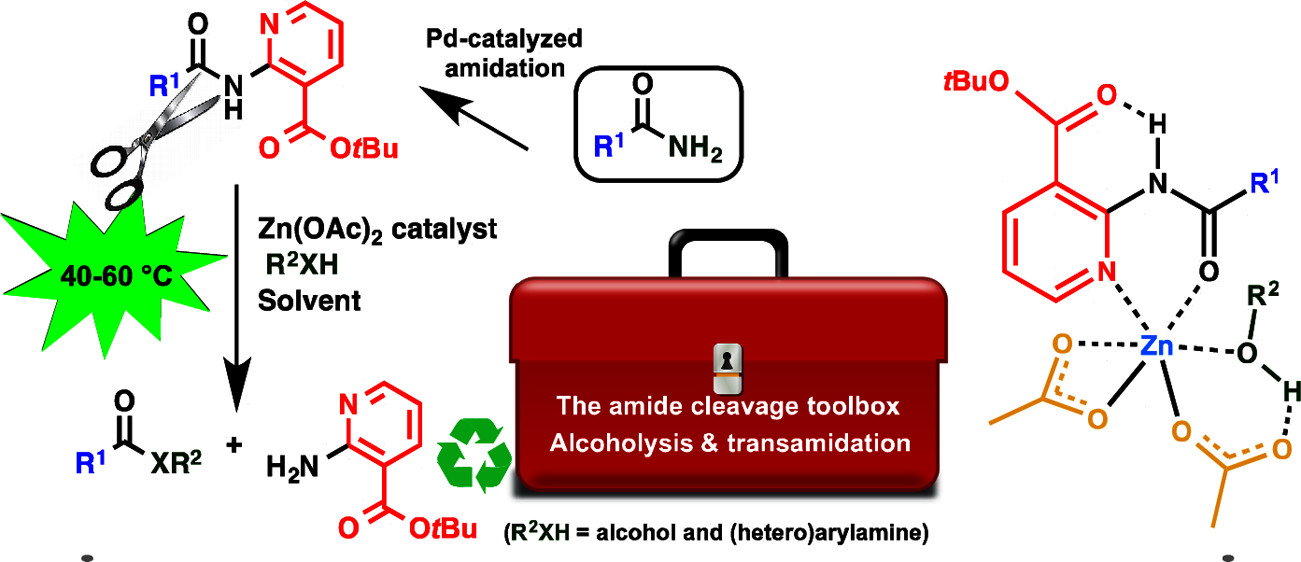
This paper was selected as the ACS Editors’ Choice!
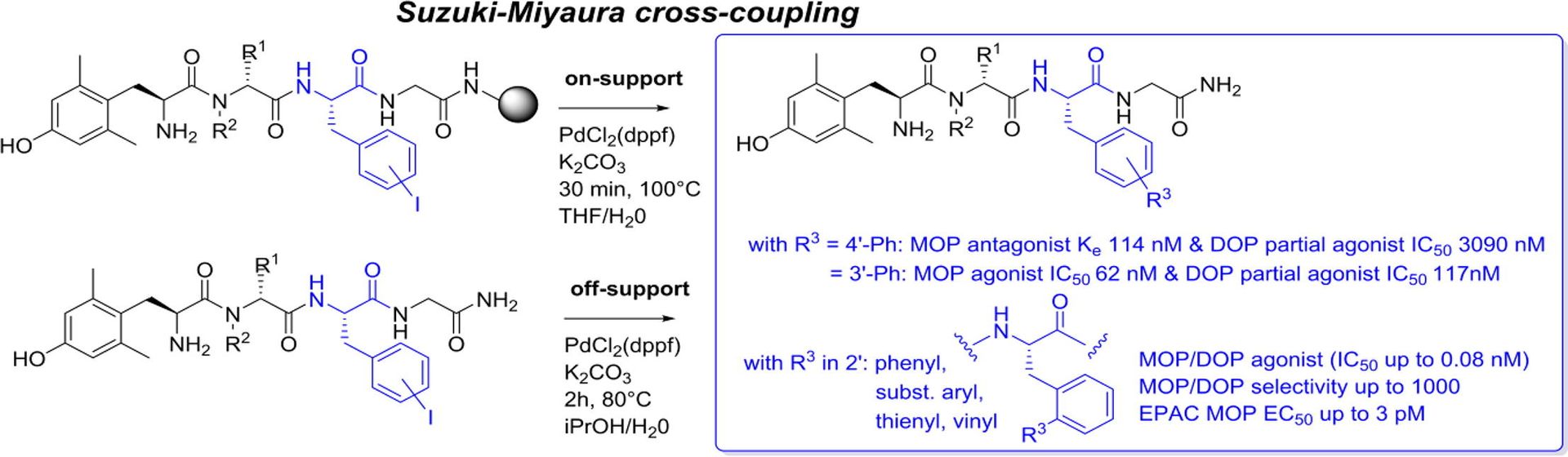
Chemical Space Screening around Phe3 in Opioid Peptides: Modulating µ versus δ Agonism by Suzuki-Miyaura Cross-Couplings
T. Willemse, E. Eiselt, K. Hollanders, W. Schepens, H. W.T. van Vlijmen, N. N. Chung, V. Blais, B. Holleran, J.-M. Longpré, P. W. Schiller, B. U. W. Maes, P. Sarret, L. Gendron, S. Ballet, Bioorganic Med. Chem. Lett. 2018, 28, 2320–2323.
A Bifunctional Biased Mu Opioid Agonist - Neuropeptide FF Receptor Antagonist as Analgesic with Improved Acute and Chronic Side Effects
A. Drieu la Rochelle, K. Guillemyn, M. Dumitrascuta, C. Martin, V. Utard, R. Quillet, S. Schneider, F. Daubeuf, T. Willemse, P. Mampuys, B. U. W. Maes, N. Frossard, F. Bihel, M. Spetea, F. Simonin, S. Ballet, Pain 2018, 159, 1705–1718.
Modular Three-Component Synthesis of 4-Aminoquinolines via an Imidoylative Sonogashira/Cyclization Cascade
J. W. Collet, K. Ackermans, J. Lambregts, B. U. W. Maes, R. V. A. Orru, Eelco Ruijter, J. Org. Chem. 2018, 83, 854–861.
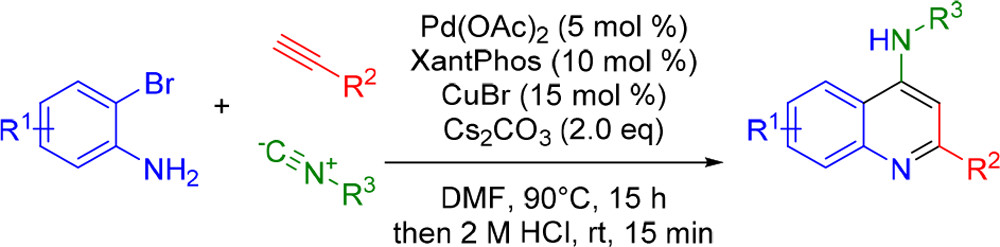
Rapid Construction of Substituted 3-amino-1,5-benzothiazepin-4(5H)-one Dipeptide Scaffolds through an Ugi-4CR – Ullmann Cross-Coupling Sequence
O. Van der Poorten, R. Van Den Hauwe, K. Hollanders, B. U. W. Maes, D. Tourwé, M. Jidaa, S. Ballet, Org. Biomol. Chem. 2018, 16, 1242–1246.
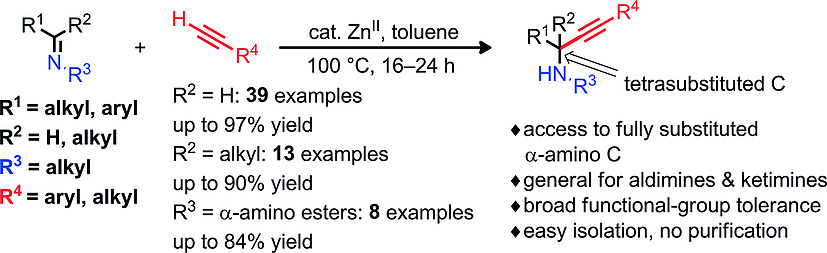
Zinc(II)-Catalyzed Synthesis of Propargylamines by Coupling Aldimines and Ketimines with Alkynes
S. A. Shehzadi, A. Saeed, F. Lemière, B. U. W. Maes, K. Abbaspour Tehrani, Eur. J. Org. Chem. 2018, 78–88.
2017
Proton Sponge Analogue of the Tröger's Base: A Compound with Remarkable Enantiomeric Stability
V. A. Ozeryanskii, M. P. Vlasenko, A. F. Pozharskii, S. Sergeyev, B. U. W. Maes, P. Franck, W. A. Herrebout, ChemistrySelect 2017, 2, 9882–9887.
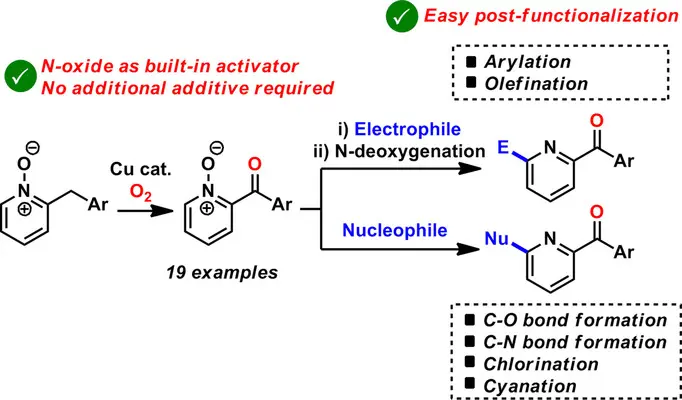
Copper-Catalyzed Aerobic Oxygenation of Benzylpyridine N-Oxides and Subsequent Post-Functionalization
H. Sterckx, C. Sambiagio, V. Médran-Navarrete, B. U. W. Maes, Adv. Synth. Catal. 2017, 359, 3226–3236.
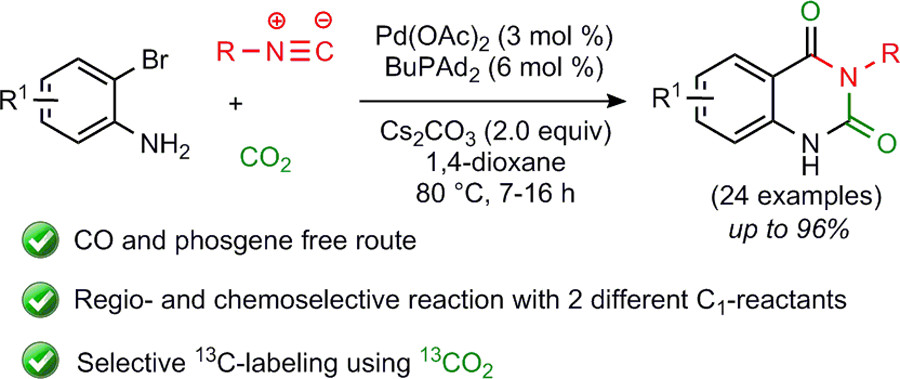
Combining Isocyanides with Carbon Dioxide in Palladium-Catalyzed Heterocycle Synthesis: N3-Substituted Quinazoline-2,4(1H,3H)-diones via a Three-Component Reaction
P. Mampuys, H. Neumann, S. Sergeyev, R. V. A. Orru, H. Jiao, A. Spannenberg, B. U. W. Maes, M. Beller, ACS Catal. 2017, 7, 5549–5556.
Electrosynthesis: A New Frontier in Aerobic Oxidation?
C. Sambiagio, H. Sterckx, B. U. W. Maes, ACS Cent. Sci. 2017, 3, 686–688.
Carbon–Nitrogen Bond Formation Through Cross-Dehydrogenative Coupling Reactions
M. Baeten, B. U. W. Maes, Advances in Organometallic Chemistry, Chapter 5, 2017, 67, 401–481.

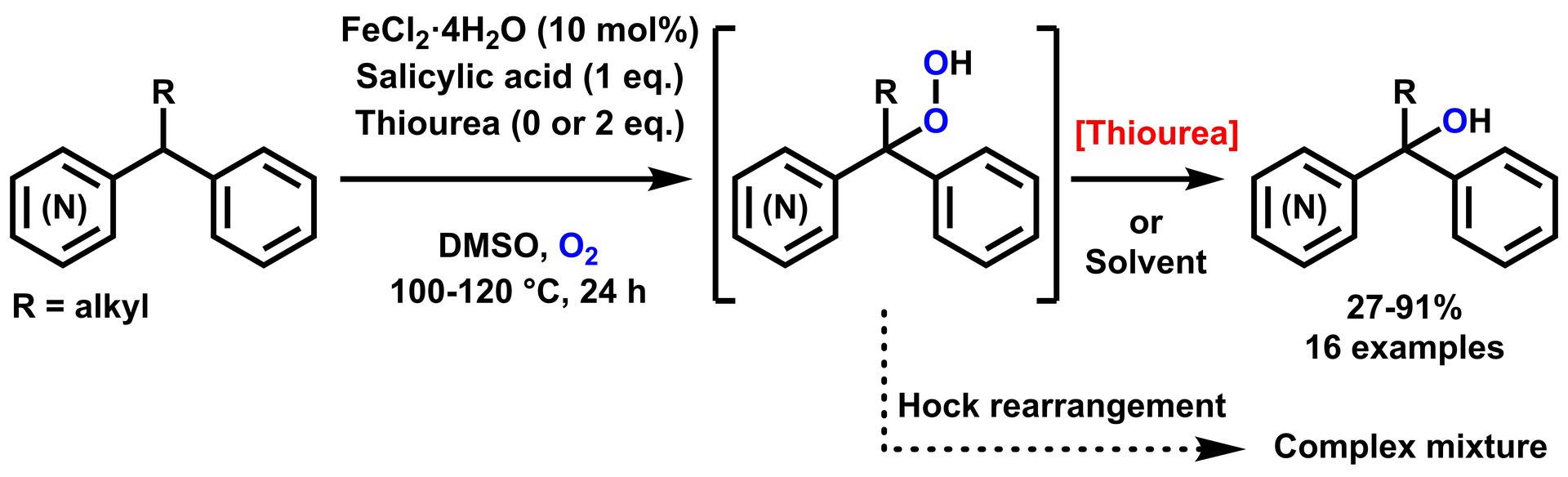
Iron-Catalyzed Aerobic Oxidation of (Alkyl)(aryl)azinylmethanes
H. Sterckx, C. Sambiagio, F. Lemière, K. A. Tehrani, B. U. W. Maes, Synlett 2017, 28, 1564–1569
Published as part of the Cluster Catalytic Aerobic Oxidations
(cluster editors: Shannon S. Stahl and Tomislav Rovis).
The Suzuki–Miyaura Cross-Coupling as a Versatile Tool for Peptide Diversification and Cyclization
T. Willemse, W. Schepens, H. W. T. van Vlijmen, B. U. W. Maes, S. Ballet, Catalysts 2017, 7, 74.
This article belongs to the Special Issue
Suzuki–Miyaura Cross-Coupling Reaction and Potential Applications.
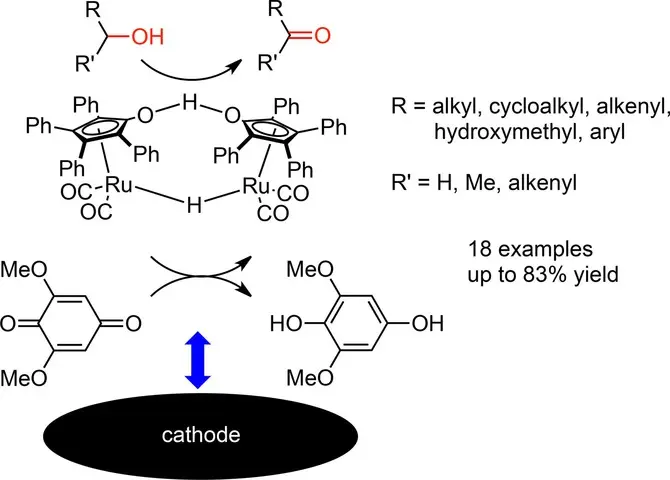
Cooperative Electrocatalytic and Chemoselective Alcohol Oxidation by Shvo's Catalyst.
J. Lybaert, S. Trashin, B. U. W. Maes, K. De Wael, K. A. Tehrani, Adv. Synth. Catal. 2017, 359, 919–925.
“Dark” Singlet Oxygen and EPR Spin Trapping as Convenient Tools to Assess Photolytic Drug Degradation
P. Persich, S. Hostyn, C. Joie, G. Winderickx, J. Pikkemaat, E. P. Romijn, B. U. W. Maes, J. Pharm. Sci. 2017, 106, 1310–1316.
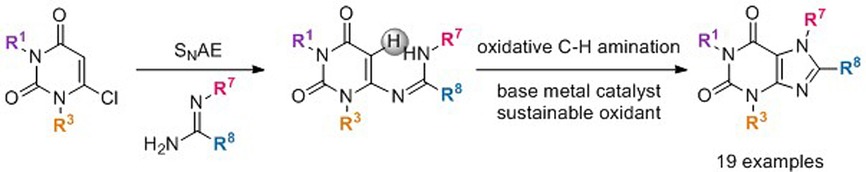
Concise Xanthine Synthesis via a Double Amidination Reaction of a 6-Chlorouracil with Amidines using Base Metal Catalysis.
B. Morel, P. Franck, J. Bidange, S. Sergeyev, D. Smith, J. Moseley, B. U. W. Maes, ChemSusChem 2017, 10, 624–628.
2016
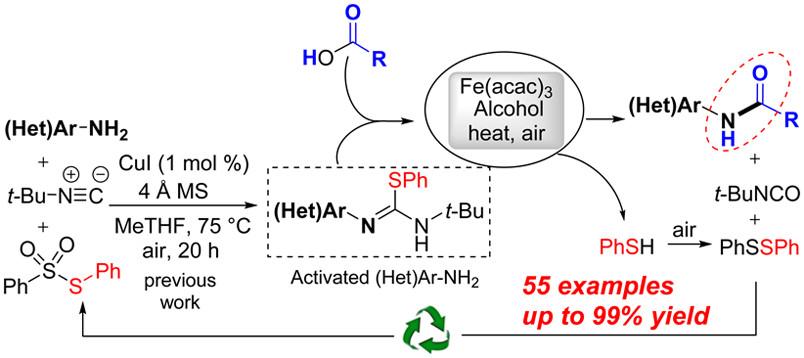
Amine Activation: Synthesis of N‐(Hetero)arylamides from Isothioureas and Carboxylic Acids.
Y.-P. Zhu, S. Sergeyev, P. Franck, R. V. A. Orru, B. U. W. Maes, Org. Lett. 2016, 18, 4602–4605.
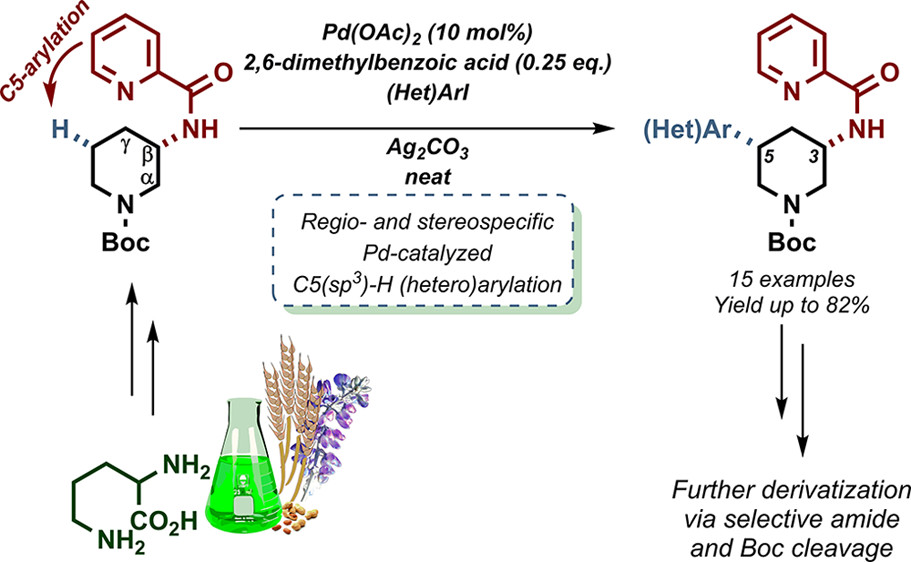
Remote Functionalization: Palladium-Catalyzed C5(sp³)-H Arylation of 1-Boc-3-aminopiperidine Through the Use of a Bidentate Directing Group.
B. F. Van Steijvoort, N. Kaval, A. A. Kulago, B. U. W. Maes, ACS Catal. 2016, 6, 4486–4490.
A Journey Through Metal-Catalyzed C-H Functionalization of Heterocycles: Insights and Trends.
J. Maes, B. U. W. Maes, Advances in Heterocyclic Chemistry, Chapter 5, 2016, 120, 137–194.

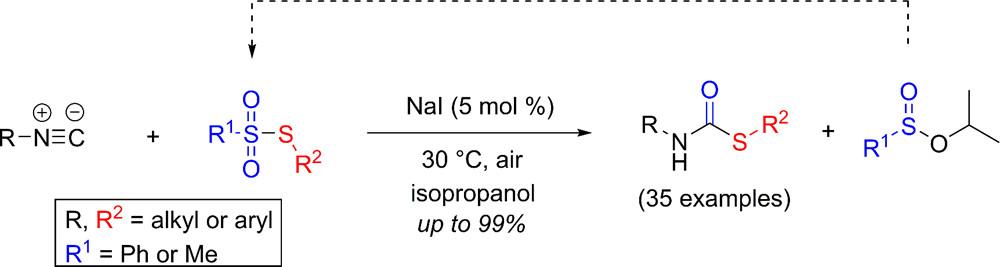
Iodide-Catalyzed Synthesis of Secondary Thiocarbamates from Isocyanides and Thiosulfonates.
P. Mampuys, Y. Zhu, S. Sergeyev, E. Ruijter, R. V. A. Orru, S. Van Doorslaer, B. U. W. Maes, Org. Lett. 2016, 18, 2808–2811.
Palladium-Catalyzed Construction of Amidines from Arylboronic Acids under Oxidative Conditions.
F. Zhu, Y. Li, Z. Wang, R. V. A. Orru, B. U. W. Maes, X.-F. Wu, Chem. Eur. J. 2016, 22, 7743–7746.
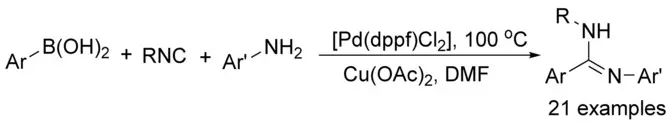
This manuscript was selected as Hot Paper!
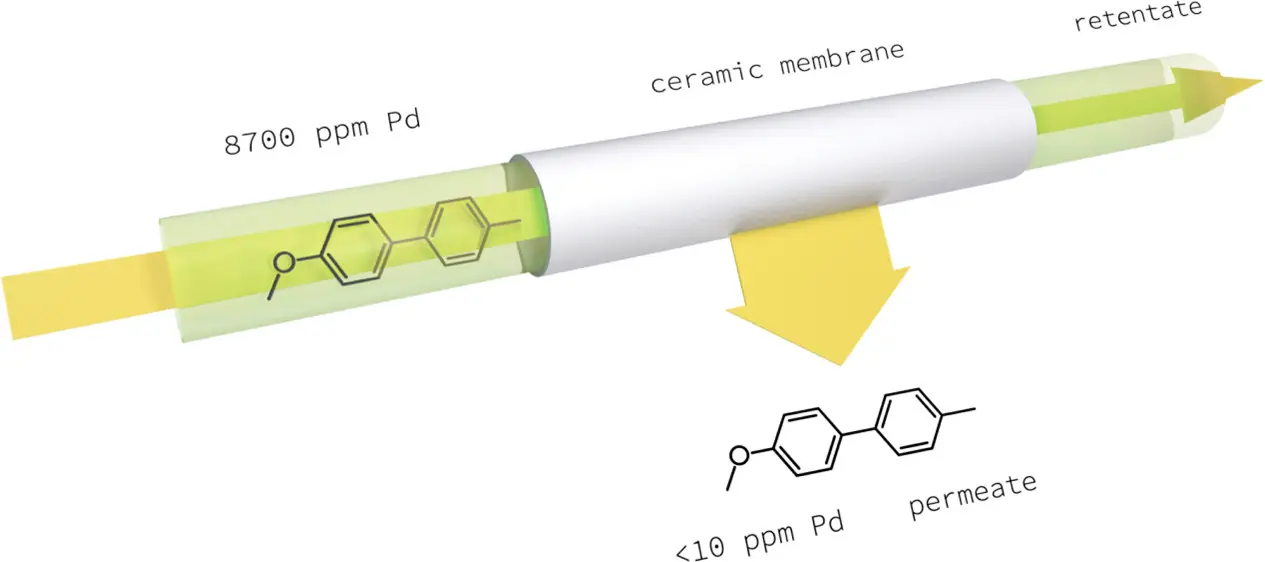
On the potential of homogeneous Pd catalyst separation by ceramic membranes. Application to down-stream and continuous flow processes.
D. Ormerod, N. Lefevre, M. Dorbec, I. Eyskens, P. Vloemans, K. Duyssens, V. Diez de la Torre, N. Kaval, E. Merkul, S. Sergeyev, B. U. W. Maes, Org. Process Res. Dev. 2016, 20, 911–920.
Base Metals in Catalysis: From Zero to Hero.
J. Maes, E. A. Mitchell, B. U. W. Maes, Green and Sustainable Medicinal Chemistry: Methods, Tools and Strategies for the 21st Century Pharmaceutical Industry, Chapter 16, 2016, 192–202.

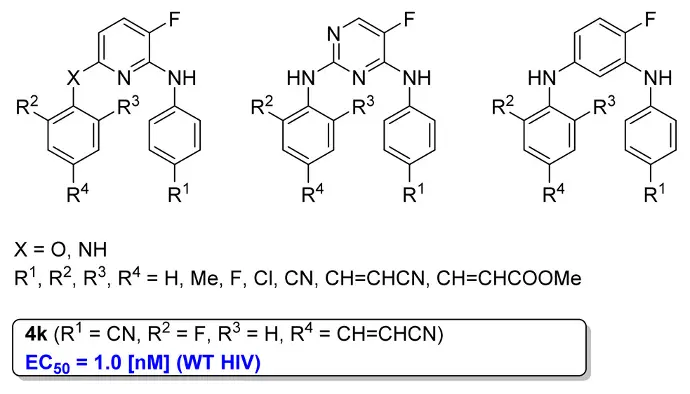
2,6-Di(Arylamino)-3-Fluoropyridine Derivatives as HIV Non-Nucleoside Reverse Transcriptase Inhibitors
S. Sergeyev, A. K. Yadav, P. Franck, J. Michiels, P. Lewi, J. Heeres, G. Vanham, K. K. Ariën, C. M. L. Vande Velde, H. De Winter, B. U. W. Maes, J. Med. Chem. 2016, 59, 1854–1868.
Base metal-catalyzed benzylic oxidation of (aryl)(heteroaryl)methanes with molecular oxygen.
H. Sterckx, J. De Houwer, C. Mensch, W. A. Herrebout, K. Abbaspour Tehrani, B. Maes, Beilstein J. Org. Chem. 2016, 12, 144–153.

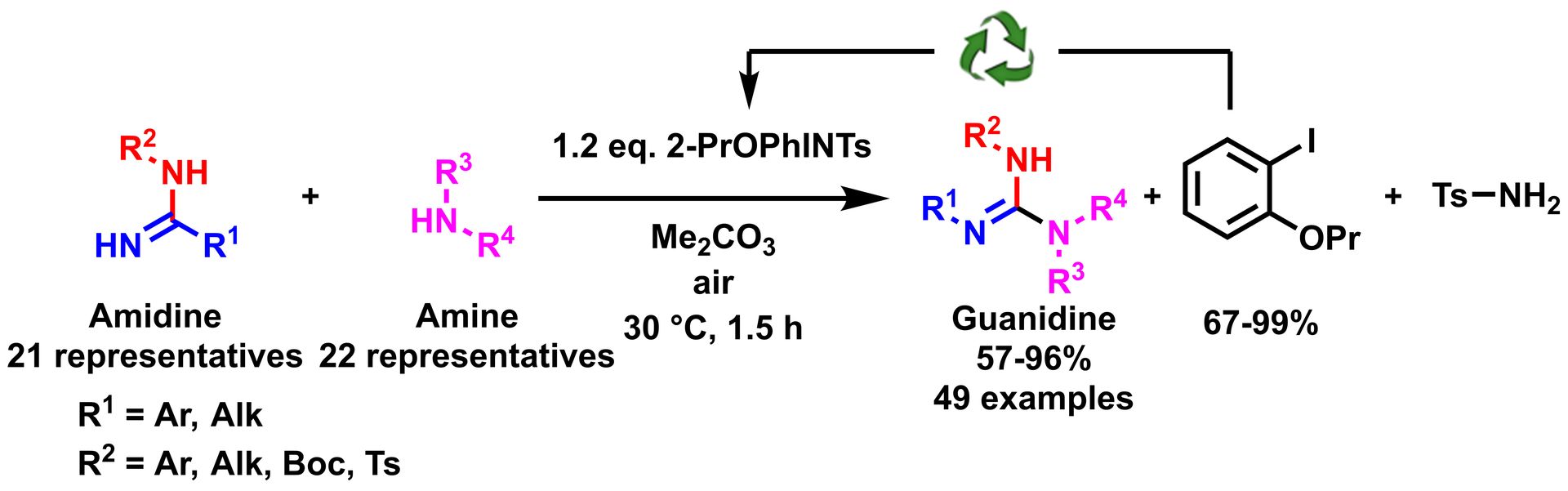
Guanidine Synthesis: Use of Amidines as Guanylating Agents.
M. Baeten, B. U. W. Maes, Adv. Synth. Catal. 2016, 358, 826–833.
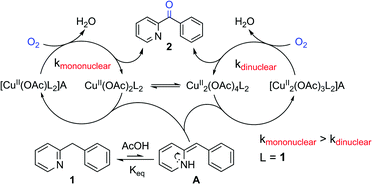
Mechanism of the Cu(II)-Catalyzed Benzylic Oxygenation of (Aryl)(heteroaryl)methanes with Oxygen.
H. Sterckx, J. De Houwer, C. Mensch, I. Caretti, K. Abbaspour Tehrani, W. A. Herrebout, S. Van Doorslaer, B. U. W. Maes, Chem. Sci. 2016, 7, 346–357.